Tissue-Resident Macrophages Promote Renal Cystic Disease
- PMID: 31337691
- PMCID: PMC6779366
- DOI: 10.1681/ASN.2018080810
Tissue-Resident Macrophages Promote Renal Cystic Disease
Abstract
Background: Mutations affecting cilia proteins have an established role in renal cyst formation. In mice, the rate of cystogenesis is influenced by the age at which cilia dysfunction occurs and whether the kidney has been injured. Disruption of cilia function before postnatal day 12-14 results in rapid cyst formation; however, cyst formation is slower when cilia dysfunction is induced after postnatal day 14. Rapid cyst formation can also be induced in conditional adult cilia mutant mice by introducing renal injury. Previous studies indicate that macrophages are involved in cyst formation, however the specific role and type of macrophages responsible has not been clarified.
Methods: We analyzed resident macrophage number and subtypes during postnatal renal maturation and after renal injury in control and conditional Ift88 cilia mutant mice. We also used a pharmacological inhibitor of resident macrophage proliferation and accumulation to determine the importance of these cells during rapid cyst formation.
Results: Our data show that renal resident macrophages undergo a phenotypic switch from R2b (CD11clo) to R2a (CD11chi) during postnatal renal maturation. The timing of this switch correlates with the period in which cyst formation transitions from rapid to slow following induction of cilia dysfunction. Renal injury induces the reaccumulation of juvenile-like R2b resident macrophages in cilia mutant mice and restores rapid cystogenesis. Loss of primary cilia in injured conditional Ift88 mice results in enhanced epithelial production of membrane-bound CSF1, a cytokine that promotes resident macrophage proliferation. Inhibiting CSF1/CSF1-receptor signaling with a CSF1R kinase inhibitor reduces resident macrophage proliferation, R2b resident macrophage accumulation, and renal cyst formation in two mouse models of cystic disease.
Conclusions: These data uncover an important pathogenic role for resident macrophages during rapid cyst progression.
Keywords: CSF1R; cilia; cystic kidney; macrophages; renal injury.
Copyright © 2019 by the American Society of Nephrology.
Figures


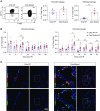
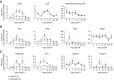

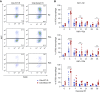

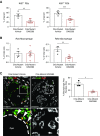
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

