Magnesium transporter 1 (MAGT1) deficiency causes selective defects in N- linked glycosylation and expression of immune-response genes
- PMID: 31337704
- PMCID: PMC6746436
- DOI: 10.1074/jbc.RA119.008903
Magnesium transporter 1 (MAGT1) deficiency causes selective defects in N- linked glycosylation and expression of immune-response genes
Abstract
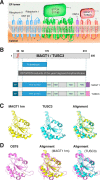
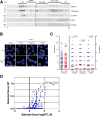
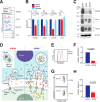
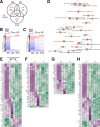
Magnesium transporter 1 (MAGT1) critically mediates magnesium homeostasis in eukaryotes and is highly-conserved across different evolutionary branches. In humans, loss-of-function mutations in the MAGT1 gene cause X-linked magnesium deficiency with Epstein-Barr virus (EBV) infection and neoplasia (XMEN), a disease that has a broad range of clinical and immunological consequences. We have previously shown that EBV susceptibility in XMEN is associated with defective expression of the antiviral natural-killer group 2 member D (NKG2D) protein and abnormal Mg2+ transport. New evidence suggests that MAGT1 is the human homolog of the yeast OST3/OST6 proteins that form an integral part of the N-linked glycosylation complex, although the exact contributions of these perturbations in the glycosylation pathway to disease pathogenesis are still unknown. Using MS-based glycoproteomics, along with CRISPR/Cas9-KO cell lines, natural killer cell-killing assays, and RNA-Seq experiments, we now demonstrate that humans lacking functional MAGT1 have a selective deficiency in both immune and nonimmune glycoproteins, and we identified several critical glycosylation defects in important immune-response proteins and in the expression of genes involved in immunity, particularly CD28. We show that MAGT1 function is partly interchangeable with that of the paralog protein tumor-suppressor candidate 3 (TUSC3) but that each protein has a different tissue distribution in humans. We observed that MAGT1-dependent glycosylation is sensitive to Mg2+ levels and that reduced Mg2+ impairs immune-cell function via the loss of specific glycoproteins. Our findings reveal that defects in protein glycosylation and gene expression underlie immune defects in an inherited disease due to MAGT1 deficiency.
Keywords: Epstein-Barr virus (EBV) infection; N-linked glycosylation (NLG) complex; X-linked magnesium deficiency with Epstein–Barr virus infection and neoplasia (XMEN); glycoprotein; glycosylation; immunodeficiency; immunology; infection; magnesium; magnesium transporter 1 (MAGT1); oligosaccharyltransferase (OST); transporter.
© 2019 Matsuda-Lennikov et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Grubbs R. D., and Maguire M. E. (1987) Magnesium as a regulatory cation: criteria and evaluation. Magnesium 6, 113–127 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

