S-Palmitoylation of junctophilin-2 is critical for its role in tethering the sarcoplasmic reticulum to the plasma membrane
- PMID: 31337710
- PMCID: PMC6737222
- DOI: 10.1074/jbc.RA118.006772
S-Palmitoylation of junctophilin-2 is critical for its role in tethering the sarcoplasmic reticulum to the plasma membrane
Abstract
Junctophilins (JPH1-JPH4) are expressed in excitable and nonexcitable cells, where they tether endoplasmic/sarcoplasmic reticulum (ER/SR) and plasma membranes (PM). These ER/SR-PM junctions bring Ca-release channels in the ER/SR and Ca as well as Ca-activated K channels in the PM to within 10-25 nm. Such proximity is critical for excitation-contraction coupling in muscles, Ca modulation of excitability in neurons, and Ca homeostasis in nonexcitable cells. JPHs are anchored in the ER/SR through the C-terminal transmembrane domain (TMD). Their N-terminal
Keywords: ER–PM junction; endoplasmic reticulum (ER); excitation–contraction coupling (E-C coupling); lipid raft; palmitoylation; plasma membrane; post-translational modification.
© 2019 Jiang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

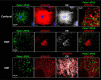


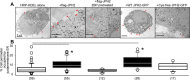


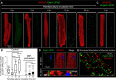
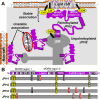
Similar articles
-
Molecular determinants of homo- and heteromeric interactions of Junctophilin-1 at triads in adult skeletal muscle fibers.Proc Natl Acad Sci U S A. 2019 Jul 30;116(31):15716-15724. doi: 10.1073/pnas.1820980116. Epub 2019 Jul 17. Proc Natl Acad Sci U S A. 2019. PMID: 31315980 Free PMC article.
-
The role of junctophilin proteins in cellular function.Physiol Rev. 2022 Jul 1;102(3):1211-1261. doi: 10.1152/physrev.00024.2021. Epub 2022 Jan 10. Physiol Rev. 2022. PMID: 35001666 Free PMC article. Review.
-
Organization of junctional sarcoplasmic reticulum proteins in skeletal muscle fibers.J Muscle Res Cell Motil. 2015 Dec;36(6):501-15. doi: 10.1007/s10974-015-9421-5. Epub 2015 Sep 15. J Muscle Res Cell Motil. 2015. PMID: 26374336 Review.
-
Interaction of the Joining Region in Junctophilin-2 With the L-Type Ca2+ Channel Is Pivotal for Cardiac Dyad Assembly and Intracellular Ca2+ Dynamics.Circ Res. 2021 Jan 8;128(1):92-114. doi: 10.1161/CIRCRESAHA.119.315715. Epub 2020 Oct 23. Circ Res. 2021. PMID: 33092464 Free PMC article.
-
Plasma membrane curvature regulates the formation of contacts with the endoplasmic reticulum.Nat Cell Biol. 2024 Nov;26(11):1878-1891. doi: 10.1038/s41556-024-01511-x. Epub 2024 Sep 17. Nat Cell Biol. 2024. PMID: 39289582 Free PMC article.
Cited by
-
Regulation of cardiomyocyte intracellular trafficking and signal transduction by protein palmitoylation.Biochem Soc Trans. 2024 Feb 28;52(1):41-53. doi: 10.1042/BST20221296. Biochem Soc Trans. 2024. PMID: 38385554 Free PMC article.
-
Altered myocardial lipid regulation in junctophilin-2-associated familial cardiomyopathies.Life Sci Alliance. 2024 Mar 4;7(5):e202302330. doi: 10.26508/lsa.202302330. Print 2024 May. Life Sci Alliance. 2024. PMID: 38438248 Free PMC article.
-
Activation of Ca transport in cardiac microsomes enriches functional sets of ER and SR proteins.Res Sq [Preprint]. 2023 Feb 8:rs.3.rs-2557992. doi: 10.21203/rs.3.rs-2557992/v1. Res Sq. 2023. Update in: Mol Cell Biochem. 2024 Jan;479(1):85-98. doi: 10.1007/s11010-023-04708-0. PMID: 36798315 Free PMC article. Updated. Preprint.
-
A Not-So-Ancient Grease History: Click Chemistry and Protein Lipid Modifications.Chem Rev. 2021 Jun 23;121(12):7178-7248. doi: 10.1021/acs.chemrev.0c01108. Epub 2021 Apr 6. Chem Rev. 2021. PMID: 33821625 Free PMC article. Review.
-
Targeting JP2: A New Treatment for Pulmonary Hypertension.Oxid Med Cell Longev. 2021 Aug 6;2021:2003446. doi: 10.1155/2021/2003446. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34394822 Free PMC article. Review.
References
-
- van Oort R. J., Garbino A., Wang W., Dixit S. S., Landstrom A. P., Gaur N., De Almeida A. C., Skapura D. G., Rudy Y., Burns A. R., Ackerman M. J., and Wehrens X. H. (2011) Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation 123, 979–988 10.1161/CIRCULATIONAHA.110.006437 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

