Neutralizing antibodies against Mayaro virus require Fc effector functions for protective activity
- PMID: 31337735
- PMCID: PMC6781005
- DOI: 10.1084/jem.20190736
Neutralizing antibodies against Mayaro virus require Fc effector functions for protective activity
Abstract
Despite causing outbreaks of fever and arthritis in multiple countries, no countermeasures exist against Mayaro virus (MAYV), an emerging mosquito-transmitted alphavirus. We generated 18 neutralizing mAbs against MAYV, 11 of which had "elite" activity that inhibited infection with EC50 values of <10 ng/ml. Antibodies with the greatest inhibitory capacity in cell culture mapped to epitopes near the fusion peptide of E1 and in domain B of the E2 glycoproteins. Unexpectedly, many of the elite neutralizing mAbs failed to prevent MAYV infection and disease in vivo. Instead, the most protective mAbs bound viral antigen on the cell surface with high avidity and promoted specific Fc effector functions, including phagocytosis by neutrophils and monocytes. In subclass switching studies, murine IgG2a and humanized IgG1 mAb variants controlled infection better than murine IgG1 and humanized IgG1-N297Q variants. An optimally protective antibody response to MAYV and possibly other alphaviruses may require tandem virus neutralization by the Fab moiety and effector functions of the Fc region.
© 2019 Earnest et al.
Figures
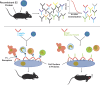
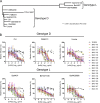
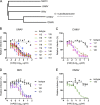



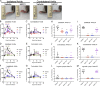

Comment in
-
Antibody barriers to going viral.J Exp Med. 2019 Oct 7;216(10):2226-2228. doi: 10.1084/jem.20191099. Epub 2019 Aug 23. J Exp Med. 2019. PMID: 31444247 Free PMC article.
References
-
- Auguste A.J., Liria J., Forrester N.L., Giambalvo D., Moncada M., Long K.C., Morón D., de Manzione N., Tesh R.B., Halsey E.S., et al. 2015. Evolutionary and ecological characterization of Mayaro virus strains isolated during an outbreak, Venezuela, 2010. Emerg. Infect. Dis. 21:1742–1750. 10.3201/eid2110.141660 - DOI - PMC - PubMed
-
- Austin S.K., Dowd K.A., Shrestha B., Nelson C.A., Edeling M.A., Johnson S., Pierson T.C., Diamond M.S., and Fremont D.H.. 2012. Structural basis of differential neutralization of DENV-1 genotypes by an antibody that recognizes a cryptic epitope. PLoS Pathog. 8:e1002930 10.1371/journal.ppat.1002930 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

