A collagen-based microwell migration assay to study NK-target cell interactions
- PMID: 31337806
- PMCID: PMC6650390
- DOI: 10.1038/s41598-019-46958-3
A collagen-based microwell migration assay to study NK-target cell interactions
Abstract
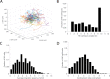
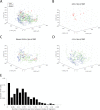
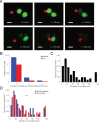
Natural killer (NK) cell cytotoxicity in tissue is dependent on the ability of NK cells to migrate through the extracellular matrix (ECM) microenvironment. Traditional imaging studies of NK cell migration and cytotoxicity have utilized 2D surfaces, which do not properly reproduce the structural and mechanical cues that shape the migratory response of NK cells in vivo. Here, we have combined a microwell assay that allows long-term imaging and tracking of small, well-defined populations of NK cells with an interstitial ECM-like matrix. The assay allows for long-term imaging of NK-target cell interactions within a confined 3D volume. We found marked differences in motility between individual cells with a small fraction of the cells moving slowly and being confined to a small volume within the matrix, while other cells moved more freely. A majority of NK cells also exhibited transient variation in their motility, alternating between periods of migration arrest and movement. The assay could be used as a complement to in vivo imaging to study human NK cell heterogeneity in migration and cytotoxicity.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

