Randomised phase 3 study of adjuvant chemotherapy with or without nadroparin in patients with completely resected non-small-cell lung cancer: the NVALT-8 study
- PMID: 31337877
- PMCID: PMC6738047
- DOI: 10.1038/s41416-019-0533-3
Randomised phase 3 study of adjuvant chemotherapy with or without nadroparin in patients with completely resected non-small-cell lung cancer: the NVALT-8 study
Abstract
Background: Retrospective studies suggest that low molecular weight heparin may delay the development of metastasis in patients with resected NSCLC.
Methods: Multicentre phase 3 study with patients with completely resected NSCLC who were randomised after surgery to receive chemotherapy with or without nadroparin. The main exclusion criteria were R1/2 and wedge/segmental resection. FDG-PET was required. The primary endpoint was recurrence-free survival (RFS).
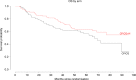
Results: Among 235 registered patients, 202 were randomised (nadroparin: n = 100; control n = 102). Slow accrual enabled a decrease in the number of patients needed from 600 to 202, providing 80% power to compare RFS with 94 events (α = 0.05; 2-sided). There were no differences in bleeding events between the two groups. The median RFS was 65.2 months (95% CI, 36-NA) in the nadroparin arm and 37.7 months (95% CI, 22.7-NA) in the control arm (HR 0.77 (95% CI, 0.53-1.13, P = 0.19). FDG-PET SUVmax ≥10 predicted a greater likelihood of recurrence in the first year (HR 0.48, 95% CI 0.22-0.9, P = 0.05).
Conclusions: Adjuvant nadroparin did not improve RFS in patients with resected NSCLC. In this study, a high SUVmax predicted a greater likelihood of recurrence in the first year.
Clinical trial registration: Netherlands Trial registry: NTR1250/1217.
Conflict of interest statement
H.J.M.G., E.F.S. and A.-M.D. were on the advisory boards of Lilly and Roche. The remaining authors authors declare no competing interests.
Figures



References
-
- Vansteenkiste JF, Stroobants SG, Dupont PJ, de Leyn PR, Verbeken EK, Deneffe GJ, et al. Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: an analysis of 125 cases. Leuven Lung cancer group. J. Clin. Oncol. 1999;17:3201–3206. doi: 10.1200/JCO.1999.17.10.3201. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

