The Antioxidant MitoQ Protects Against CSE-Induced Endothelial Barrier Injury and Inflammation by Inhibiting ROS and Autophagy in Human Umbilical Vein Endothelial Cells
- PMID: 31337974
- PMCID: PMC6643142
- DOI: 10.7150/ijbs.30193
The Antioxidant MitoQ Protects Against CSE-Induced Endothelial Barrier Injury and Inflammation by Inhibiting ROS and Autophagy in Human Umbilical Vein Endothelial Cells
Abstract
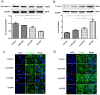
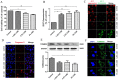
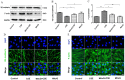
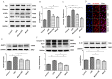
Chronic obstructive pulmonary disease (COPD) is a common disease characterized by persistent airflow limitation. Pulmonary vascular endothelial barrier injury and inflammation are increasingly considered to be important pathophysiological processes in cigarette smoke extract (CSE)-induced COPD, but the mechanism remains unclear. To identify the cellular mechanism of endothelial barrier injury and inflammation in CSE-treated human umbilical vein endothelial cells (HUVECs), we investigated the effect of the mitochondrion-targeting antioxidant mitoquinone (MitoQ) on endothelial barrier injury and inflammation. We demonstrated that MitoQ restored endothelial barrier integrity by preventing VE-cadherin disassembly and actin cytoskeleton remodeling, as well as decreased inflammation by the NF-κB and NLRP3 inflammasome pathways in endothelial cells. In addition, MitoQ also maintained mitochondrial function by reducing the production of ROS and excess autophagy. Inhibition of autophagy by 3-MA protected against cytotoxicity that was induced by CSE in HUVECs. Overall, our study indicated that mitochondrial damage is a key promoter in the induction of endothelial barrier dysfunction and inflammation by CSE. The protective effect of MitoQ is related to the inhibition of ROS and excess autophagy in CSE-induced HUVEC injury.
Keywords: Autophagy; Chronic obstructive pulmonary disease; Cigarette smoke extract; Endothelial barrier; Inflammation; ROS.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Lebowitz MD. Smoking habits and changes in smoking habits as they relate to chronic conditions and respiratory symptoms. American journal of epidemiology. 1977;105:534–43. - PubMed
-
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. American journal of respiratory and critical care medicine. 2001;163:1256–76. - PubMed
-
- Auerbach O, Hammond EC, Garfinkel L, Benante C. Relation of smoking and age to emphysema. Whole-lung section study. The New England journal of medicine. 1972;286:853–7. - PubMed
-
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP. et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

