Prevalence of Anti-Adeno-Associated Virus Immune Responses in International Cohorts of Healthy Donors
- PMID: 31338384
- PMCID: PMC6629972
- DOI: 10.1016/j.omtm.2019.05.014
Prevalence of Anti-Adeno-Associated Virus Immune Responses in International Cohorts of Healthy Donors
Abstract
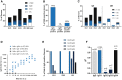
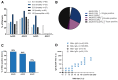
Preexisting immunity against adeno-associated virus (AAV) is a major challenge facing AAV gene therapy, resulting in the exclusion of patients from clinical trials. Accordingly, proper assessment of anti-AAV immunity is necessary for understanding clinical data and for product development. Previous studies on anti-AAV prevalence lack method standardization, rendering the assessment of prevalence difficult. Addressing this need, we used clinical assays that were validated according to guidelines for a comprehensive characterization of anti-AAV1, -AAV2, -AAV5, and -AAV8 immunity in large international cohorts of healthy donors and patients with hemophilia B. Here, we report a higher than expected average prevalence for anti-AAV8 (∼40%) and anti-AAV5 (∼30%) neutralizing antibodies (NAbs), which is supported by strongly correlating anti-AAV IgG antibody titers. A similar anti-AAV8 NAb prevalence was observed in hemophilia B patients. In addition, a high co-prevalence of NAbs against other serotypes makes switching to gene therapy using another serotype difficult. As anti-AAV T cell responses are believed to influence transduction, we characterized anti-AAV T cell responses using interleukin-2 (IL-2) and interferon-γ (IFN-γ) ELISpot assays, revealing a similar prevalence of IFN-γ responses (∼20%) against different serotypes that did not correlate with NAbs. These data, along with the long-term stability of NAbs, emphasize the need to develop strategies to circumvent anti-AAV immunity.
Keywords: AAV; adeno-associated virus; anti-AAV T cell response; anti-AAV antibody response; anti-AAV prevalence; clinical assays; gene therapy; international cohorts.
Figures



References
-
- Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

