Liraglutide treatment reduced interleukin-6 in adults with type 1 diabetes but did not improve established autonomic or polyneuropathy
- PMID: 31338868
- PMCID: PMC6848951
- DOI: 10.1111/bcp.14063
Liraglutide treatment reduced interleukin-6 in adults with type 1 diabetes but did not improve established autonomic or polyneuropathy
Abstract
Aims: Type 1 diabetes can be complicated with neuropathy that involves immune-mediated and inflammatory pathways. Glucagon-like peptide-1 receptor agonists such as liraglutide, have shown anti-inflammatory properties, and thus we hypothesized that long-term treatment with liraglutide induced diminished inflammation and thus improved neuronal function.
Methods: The study was a randomized, double-blinded, placebo-controlled trial of adults with type 1 diabetes and confirmed symmetrical polyneuropathy. They were randomly assigned (1:1) to receive either liraglutide or placebo. Titration was 6 weeks to 1.2-1.8 mg/d, continuing for 26 weeks. The primary endpoint was change in latency of early brain evoked potentials. Secondary endpoints were changes in proinflammatory cytokines, cortical evoked potential, autonomic function and peripheral neurophysiological testing.
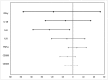
Results: Thirty-nine patients completed the study, of whom 19 received liraglutide. In comparison to placebo, liraglutide reduced interleukin-6 (-22.6%; 95% confidence interval [CI]: -38.1, -3.2; P = .025) with concomitant numerical reductions in other proinflammatory cytokines. However neuronal function was unaltered at the central, autonomic or peripheral level. Treatment was associated with -3.38 kg (95% CI: -5.29, -1.48; P < .001] weight loss and a decrease in urine albumin/creatinine ratio (-40.2%; 95% CI: -60.6, -9.5; P = .02).
Conclusion: Hitherto, diabetic neuropathy has no cure. Speculations can be raised whether mechanism targeted treatment, e.g. lowering the systemic level of proinflammatory cytokines may lead to prevention or treatment of the neuroinflammatory component in early stages of diabetic neuropathy. If ever successful, this would serve as an example of how fundamental mechanistic principles are translated into clinical practice similar to those applied in the cardiovascular and nephrological clinic.
Trial registration: ClinicalTrials.gov NCT02138045.
Keywords: anti-inflammation; diabetic neuropathy; glucagon-like peptide-1 agonist; interleukin-6; liraglutide.
© 2019 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The study sponsor was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

