Protein hydrolysate versus standard formula for preterm infants
- PMID: 31339557
- PMCID: PMC6653062
- DOI: 10.1002/14651858.CD012412.pub3
Protein hydrolysate versus standard formula for preterm infants
Abstract
Background: When human milk is not available for feeding preterm infants, protein hydrolysate, rather than standard cow's milk formulas (with intact proteins), is often used because it is perceived as being tolerated better and less likely to lead to complications. However, protein hydrolysate formulas are more expensive than standard formulas, and concern exists that their use in practice is not supported by high-quality evidence.
Objectives: To assess the effects of feeding preterm infants hydrolysed formula (vs standard cow's milk formula) on risk of feed intolerance, necrotising enterocolitis, and other morbidity and mortality.
Search methods: We used the standard Cochrane Neonatal search strategy including electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 1), in the Cochrane Library; Ovid MEDLINE (1966 to 28 January 2019); Ovid Embase (1980 to 28 January 2019); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (28 January 2019), as well as conference proceedings and previous reviews.
Selection criteria: Randomised and quasi-randomised controlled trials that compared feeding preterm infants protein hydrolysate versus standard (non-hydrolysed) cow's milk formula.
Data collection and analysis: Two review authors assessed trial eligibility and risk of bias and extracted data independently. We analysed treatment effects as described in the individual trials and reported risk ratios and risk differences for dichotomous data, and mean differences for continuous data, with respective 95% confidence intervals (CIs). We used a fixed-effect model in meta-analyses and explored potential causes of heterogeneity in sensitivity analyses. We assessed quality of evidence at the outcome level using the GRADE approach.
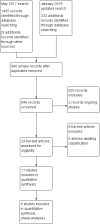
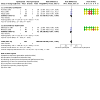
Main results: We identified 11 trials for inclusion in the review. All trials were small (total participants 665) and had various methodological limitations including uncertainty about methods to ensure allocation concealment and blinding. Most participants were clinically stable preterm infants of less than about 34 weeks' gestational age or with birth weight less than about 1750 g. Fewer participants were extremely preterm, extremely low birth weight, or growth restricted. Most trials found no effects on feed intolerance, assessed variously as mean pre-feed gastric residual volume, incidence of abdominal distension or other gastrointestinal signs of concern, or time taken to achieve full enteral feeds (meta-analysis was limited because studies used different measures). Meta-analysis showed no effect on the risk of necrotising enterocolitis (typical risk ratio 1.10, 95% CI 0.36 to 3.34; risk difference 0.00, 95% CI -0.03 to 0.04; 5 trials, 385 infants) (low-certainty evidence; downgraded for imprecision and design weaknesses).
Authors' conclusions: The identified trials provide only low-certainty evidence about the effects of feeding preterm infants protein hydrolysate versus standard formula. Existing data do not support conclusions that feeding protein hydrolysate affects the risk of feed intolerance or necrotising enterocolitis. Additional large, pragmatic trials are needed to provide more reliable and precise estimates of effectiveness and cost-effectiveness.
Conflict of interest statement
NDE declares the following: receiving a research grant award for an RCT of breast milk products by Prolacta Bioscience, 2017; receiving a grant from Danone Early Life Nutrition to support a study on feeding in late and moderately preterm infants, 2018; receiving a grant from Nestle Nutrition for transcriptomic analyses of gut tissue, 2016; lectures with Wyeth Nutrition in 2017, Nestle Nutrition Institute in 2017 and 2018, Philipps in 2017, and Fresenius, 2017.
DN has no interest to declare.
JK has no interest to declare.
WM has no interest to declare.
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor has no input on the content of the review or the editorial process (see Sources of support).
To maintain the utmost editorial independence for this Cochrane Review, an editor outside of the Cochrane Neonatal core editorial team who is not receiving any financial remuneration from the grant, Jann Foster, was the Sign‐off Editor for this review. Additionally, a Senior Editor from the Cochrane Children and Families Network, Robert Boyle, assessed and signed off on this Cochrane Review.
Figures












Update of
-
Protein hydrolysate versus standard formula for preterm infants.Cochrane Database Syst Rev. 2017 Oct 2;10(10):CD012412. doi: 10.1002/14651858.CD012412.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Jul 24;7:CD012412. doi: 10.1002/14651858.CD012412.pub3. PMID: 28968486 Free PMC article. Updated.
Similar articles
-
Protein hydrolysate versus standard formula for preterm infants.Cochrane Database Syst Rev. 2017 Oct 2;10(10):CD012412. doi: 10.1002/14651858.CD012412.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Jul 24;7:CD012412. doi: 10.1002/14651858.CD012412.pub3. PMID: 28968486 Free PMC article. Updated.
-
Formula versus donor breast milk for feeding preterm or low birth weight infants.Cochrane Database Syst Rev. 2019 Jul 19;7(7):CD002971. doi: 10.1002/14651858.CD002971.pub5. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2024 Sep 6;9:CD002971. doi: 10.1002/14651858.CD002971.pub6. PMID: 31322731 Free PMC article. Updated.
-
High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants.Cochrane Database Syst Rev. 2021 Mar 9;3(3):CD012413. doi: 10.1002/14651858.CD012413.pub3. Cochrane Database Syst Rev. 2021. PMID: 33733486 Free PMC article.
-
Re-feeding versus discarding gastric residuals to improve growth in preterm infants.Cochrane Database Syst Rev. 2019 Jul 8;7(7):CD012940. doi: 10.1002/14651858.CD012940.pub2. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2023 Jun 30;6:CD012940. doi: 10.1002/14651858.CD012940.pub3. PMID: 31283000 Free PMC article. Updated.
-
Nutrient-enriched formula versus standard formula for preterm infants.Cochrane Database Syst Rev. 2019 Jul 17;7(7):CD004204. doi: 10.1002/14651858.CD004204.pub3. Cochrane Database Syst Rev. 2019. PMID: 31314903 Free PMC article.
Cited by
-
[Clinical guidelines for the diagnosis and treatment of feeding intolerance in preterm infants (2020)].Zhongguo Dang Dai Er Ke Za Zhi. 2020 Oct;22(10):1047-1055. doi: 10.7499/j.issn.1008-8830.2008132. Zhongguo Dang Dai Er Ke Za Zhi. 2020. PMID: 33059799 Free PMC article. Chinese.
-
Human Milk Feeding for Septic Newborn Infants Might Minimize Their Exposure to Ventilation Therapy.Children (Basel). 2022 Sep 22;9(10):1450. doi: 10.3390/children9101450. Children (Basel). 2022. PMID: 36291386 Free PMC article.
-
[Clinical guidelines for the diagnosis and treatment of neonatal necrotizing enterocolitis (2020)].Zhongguo Dang Dai Er Ke Za Zhi. 2021 Jan;23(1):1-11. doi: 10.7499/j.issn.1008-8830.2011145. Zhongguo Dang Dai Er Ke Za Zhi. 2021. PMID: 33476530 Free PMC article. Chinese.
-
The effect of milk type and fortification on the growth of low-birthweight infants: An umbrella review of systematic reviews and meta-analyses.Matern Child Nutr. 2021 Jul;17(3):e13176. doi: 10.1111/mcn.13176. Epub 2021 Mar 17. Matern Child Nutr. 2021. PMID: 33733580 Free PMC article.
-
Early versus late fortification of breast milk in preterm infants: a systematic review and meta-analysis.Eur J Pediatr. 2020 Jul;179(7):1057-1068. doi: 10.1007/s00431-020-03677-6. Epub 2020 May 27. Eur J Pediatr. 2020. PMID: 32458060
References
References to studies included in this review
Baldassarre 2017 {published and unpublished data}
-
- Baldassarre ME, Di Mauro A, Fanelli M, Montagna O, Wampler J, Cooper T, et al. Shorter time to full enteral feedings among infants fed an intact protein (IP) vs an extensively hydrolyzed (EH) formula does not appear to be related to differences in gastric emptying. In: ESPGHAN. Vol. 64. 2017:920-1.
Florendo 2009 {published data only}
Huston 1992 {published data only}
-
- Huston RK, Murphy DL, Jelen BJ. Preliminary evaluation of casein hydrolysate containing formulas in low birthweight preterm infants. Pediatric Research 1992;31:289A.
Maggio 2005 {published data only}
-
- Maggio L, Zuppa AA, Sawatzki G, Valsasina R, Schubert W, Tortorolo G. Higher urinary excretion of essential amino acids in preterm infants fed protein hydrolysates. Acta Paediatrica 2005;94(1):75-84. [PMID: ] - PubMed
Mihatsch 2002 {published data only}
-
- Mihatsch WA, Franz AR, Hogel J, Pohlandt F. Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics 2002;110(6):1199-203. [PMID: ] - PubMed
Pauls 1996 {published data only}
-
- Pauls J, Bauer K, Versmold H. Randomized controlled trial of formulas with hydrolyzed versus non-hydrolyzed protein for starting enteral feedings in preterm infants < 1500g body weight. Journal of Pediatric Gastroenterology and Nutrition 1996;22(4):450.
Picaud 2001 {published data only}
-
- Picaud JC, Rigo J, Normand S, Lapillonne A, Reygrobellet B, Claris O, et al. Nutritional efficacy of preterm formula with a partially hydrolyzed protein source: a randomized pilot study. Journal of Pediatric Gastroenterology and Nutrition 2001;32(5):555-61. [PMID: ] - PubMed
Raupp 1995 {published data only}
-
- Raupp P, Von Kries R, Gobel C, Fox A, Lemburg P, Schmidt E, et al. Bone density and biochemical parameters of bone mineral metabolism and acid load in preterm infants. Cow's milk formula versus hydrolysed protein preparation. Monatsschrift fur Kinderheilkunde 1995;143(7 Suppl 2):S140-6.
Riezzo 2001 {published data only}
-
- Riezzo G, Indrio F, Montagna O, Tripaldi C, Laforgia N, Chiloiro M, et al. Gastric electrical activity and gastric emptying in preterm newborns fed standard and hydrolysate formulas. Journal of Pediatric Gastroenterology and Nutrition 2001;33(3):290-5. [PMID: ] - PubMed
Schweizer 1993 {published data only}
-
- Schweizer P, Stock GJ, Diekmann L, Heitmann F, Kalhoff H, Manz F. Build up nutrition in premature babies: premature baby milk nutrition vs protein hydrolysate [Nährungsaufbau bei Frühgeborenen (FG; GG 750 - 1250 g): FG milchahrung vs proteinhydrolysat]. Monatsschrift Kinderheilkunde 1993;141:S8.
Szajewska 2004 {published data only}
-
- Szajewska H, Mrukowicz JZ, Stoinska B, Prochowska A. Extensively and partially hydrolysed preterm formulas in the prevention of allergic diseases in preterm infants: a randomized, double-blind trial. Acta Paediatrica 2004;93(9):1159-65. [PMID: ] - PubMed
References to studies excluded from this review
Agosti 2003 {published data only}
-
- Agosti M, Pugni L, Ramenghi LA, Mosca F, Marini A. Hydrolysed proteins in preterm formula: influence on plasma aminoacids, blood fatty acids and insulinaemia. Acta Paediatrica Supplement 2003;91(441):34-8. [PMID: ] - PubMed
Corvaglia 2013 {published data only}
Logarajaha 2015 {published data only}
-
- Logarajaha V, Onga C, Jayagobib PA, Khoob PC, Heina M, Fanga H, et al. PP-15: the effect of extensively hydrolyzed protein formula in preterm infants with symptomatic gastro-oesophageal reflux. Journal of Pediatric Gastroenterology and Nutrition 2015;61(4):526. [DOI: 10.1097/01.mpg.0000472243.59979.4a] [PMID: ] - DOI - PubMed
Mihatsch 1999 {published data only}
-
- Mihatsch WA, Pohlandt F. Protein hydrolysate formula maintains homeostasis of plasma amino acids in preterm infants. Journal of Pediatric Gastroenterology and Nutrition 1999;29(4):406-10. [PMID: ] - PubMed
Mihatsch 2001a {published data only}
-
- Mihatsch WA, Hogel J, Pohlandt F. Hydrolysed protein accelerates the gastrointestinal transport of formula in preterm infants. Acta Paediatrica 2001;90(2):196-8. [PMID: ] - PubMed
Rigo 1994 {published data only}
-
- Rigo J, Salle BL, Putet G, Senterre J. Nutritional evaluation of various protein hydrolysate formulae in term infants during the first month of life. Acta Paediatrica Supplement 1994;402:100-4. [PMID: ] - PubMed
-
- Rigo J, Senterre J. Metabolic balance studies and plasma amino acid concentrations in preterm infants fed experimental protein hydrolysate preterm formulas. Acta Paediatrica 1994;405:98-104. [PMID: ] - PubMed
Rigo 1995 {published data only}
-
- Rigo J, Salle BL, Picaud JC, Putet G, Senterre J. Nutritional evaluation of protein hydrolysate formulas. European Journal of Clinical Nutrition 1995;49(Suppl 1):S26-38. [PMID: ] - PubMed
Yu 2014 {published data only}
-
- Yu MX, Zhuang SQ, Wang DH, Zhou XY, Liu XH, Shi LP, et al. Effects of extensively hydrolyzed protein formula on feeding and growth in preterm infants: a multicenter controlled clinical study. Zhongguo Dang Dai er Ke Za Zhi = Chinese Journal of Contemporary Pediatrics 2014;16(7):684-90. [PMID: ] - PubMed
References to studies awaiting assessment
del Moral 2017 {published data only}
-
- https://clinicaltrialsgov/show/NCT01156493.
Dobryanskyy 2015 {published data only}
-
- Dobryanskyy D, Borysiuk O, Novikova O, Dubrovna Y, Salabay Z, Detsyk O. Clinical effectiveness of hydrolysed protein preterm formula in prevention of feeding intolerance in very low birth weight infants. Journal of Pediatric and Neonatal Individualized Medicine 2015;4(2):e040210. [DOI: 10.7363/040210] - DOI
Gu 2017 {published data only}
Kwinta 2002 {published data only}
-
- Kwinta P, Mitkowska Z, Kruczek P, Tomasik T, Pietrzyk JJ. Influence of the lactose free and lactose containing diet on prevalence of gram-negative sepsis and feeding intolerance in very low birth weight infants: double-blind randomized trial [Wplyw rodzaju stosowanej diety na tolerancje karmienia i czestosc posocznicy Gram ujemnej u noworodkow z bardzo niska urodzeniowa masa ciala]. Przeglad Lekarski 2002;59 Suppl 1:63-6. [PMID: ] - PubMed
-
- Kwinta P, Sawiec P, Klimek M, Lis G, Cichocka-Jarosz E, Pietrzyk JJ. Correlation between early neonatal diet and atopic symptoms up to 5-7 years of age in very low birth weight infants: follow-up of randomized, double-blind study. Pediatric Allergy and Immunology: official publication of the European Society of Pediatric Allergy and Immunology 2009;20(5):458-66. - PubMed
Luo 2016 {published data only}
-
- Luo Z, Wang Y, Wang l. A study on the effects of extensively hydrolyzed formula for very/extremely low birth weight infants. Chinese Journal of Neonatology 2016;31(2):110-4.
References to ongoing studies
ACTRN12613000481774 {unpublished data only}
-
- ACTRN12613000481774. Improvement of feeding tolerance by using a new formula in preterm neonates [Effects of a new hydrolyzed powdered formula on feeding tolerance in preterm neonates: a randomized placebo-controlled study]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363880 (first received 30 April 2013).
Additional references
AAP 2012
Agostoni 2010
-
- Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):85-91. [DOI: 10.1097/MPG.0b013e3181adaee0] [PMID: ] - DOI - PubMed
Arslanoglu 2013
BNFC 2016
-
- Paediatric Formulary Committee. www.medicinescomplete. London (UK): British Medical Association, Royal Pharmaceutical Society, the Royal College of Paediatrics and Child Health, and the Neonatal and Paediatric Pharmacists Group, 2016.
Boyle 2016
Brandt 2003
Cleminson 2015
Cooke 2016
-
- Cooke RJ. Improving growth in preterm infants during initial hospital stay: principles into practice. Archives of Disease in Childhood. Fetal and Neonatal Edition 2016;101(4):F366-70. [DOI: 10.1136/archdischild-2015-310097] [PMID: ] - PubMed
Embleton 2001
-
- Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics 2001;107(2):270-3. [PMID: ] - PubMed
Embleton 2013a
Embleton 2013b
Ewer 1994
Fewtrell 1999
-
- Fewtrell M, Lucas A. Nutritional physiology: dietary requirements of term and preterm infants. In: Rennie JM, Roberton NRC, editors(s). Textbook of Neonatology. 3rd edition. Edinburgh: Churchill Livingstone, Inc., 1999:305-25.
Foucard 2005
-
- Foucard T. Is there any role for protein hydrolysates to premature newborns? Acta Paediatrica 2005;94(1):20-2. [PMID: ] - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 26 April 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014.Available at gradepro.org.
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Imdad 2013
Klingenberg 2012
Lapillonne 2016
-
- Lapillonne A, Matar M, Adleff A, Chbihi M, Kermorvant-Duchemin E, Campeotto F. Use of extensively hydrolysed formula for refeeding neonates postnecrotising enterocolitis: a nationwide survey-based, cross-sectional study. BMJ Open 2016;6(7):e008613. [DOI: 10.1136/bmjopen-2015-008613] [PMID: ] - DOI - PMC - PubMed
Leppanen 2014
Lindberg 1998
-
- Lindberg T, Engberg S, Sjoberg LB, Lonnerdal B. In vitro digestion of proteins in human milk fortifiers and in preterm formula. Journal of Pediatric Gastroenterology and Nutrition 1998;27(1):30-6. [PMID: ] - PubMed
Mihatsch 2001b
-
- Mihatsch WA, Hogel J, Pohlandt F. Hydrolysed protein accelerates the gastrointestinal transport of formula in preterm infants. Acta Paediatrica 2001;90(2):196-8. [PMID: ] - PubMed
Morgan 2011
Oldaeus 1997
Osborn 2017
Pike 2012
-
- Pike K, Brocklehurst P, Jones D, Kenyon S, Salt A, Taylor D, et al. Outcomes at 7 years for babies who developed neonatal necrotising enterocolitis: the ORACLE Children Study. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(5):F318-22. [DOI: 10.1136/fetalneonatal-2011-300244] [PMID: ] - DOI - PubMed
Quigley 2014
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). GRADE handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.gradepro.org/app/handbook/handbook.html..
Senterre 2016
Uribarri 2015
Walsh 1986
Yee 2012
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

