A hidden human proteome encoded by 'non-coding' genes
- PMID: 31340039
- PMCID: PMC6735797
- DOI: 10.1093/nar/gkz646
A hidden human proteome encoded by 'non-coding' genes
Abstract
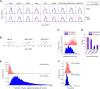
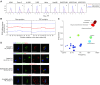
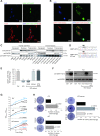
It has been a long debate whether the 98% 'non-coding' fraction of human genome can encode functional proteins besides short peptides. With full-length translating mRNA sequencing and ribosome profiling, we found that up to 3330 long non-coding RNAs (lncRNAs) were bound to ribosomes with active translation elongation. With shotgun proteomics, 308 lncRNA-encoded new proteins were detected. A total of 207 unique peptides of these new proteins were verified by multiple reaction monitoring (MRM) and/or parallel reaction monitoring (PRM); and 10 new proteins were verified by immunoblotting. We found that these new proteins deviated from the canonical proteins with various physical and chemical properties, and emerged mostly in primates during evolution. We further deduced the protein functions by the assays of translation efficiency, RNA folding and intracellular localizations. As the new protein UBAP1-AST6 is localized in the nucleoli and is preferentially expressed by lung cancer cell lines, we biologically verified that it has a function associated with cell proliferation. In sum, we experimentally evidenced a hidden human functional proteome encoded by purported lncRNAs, suggesting a resource for annotating new human proteins.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Cech T.R., Steitz J.A.. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014; 157:77–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

