Partners in Mischief: Functional Networks of Heat Shock Proteins of Plasmodium falciparum and Their Influence on Parasite Virulence
- PMID: 31340488
- PMCID: PMC6681276
- DOI: 10.3390/biom9070295
Partners in Mischief: Functional Networks of Heat Shock Proteins of Plasmodium falciparum and Their Influence on Parasite Virulence
Abstract
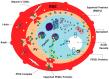
The survival of the human malaria parasite Plasmodium falciparum under the physiologically distinct environments associated with their development in the cold-blooded invertebrate mosquito vectors and the warm-blooded vertebrate human host requires a genome that caters to adaptability. To this end, a robust stress response system coupled to an efficient protein quality control system are essential features of the parasite. Heat shock proteins constitute the main molecular chaperone system of the cell, accounting for approximately two percent of the malaria genome. Some heat shock proteins of parasites constitute a large part (5%) of the 'exportome' (parasite proteins that are exported to the infected host erythrocyte) that modify the host cell, promoting its cyto-adherence. In light of their importance in protein folding and refolding, and thus the survival of the parasite, heat shock proteins of P. falciparum have been a major subject of study. Emerging evidence points to their role not only being cyto-protection of the parasite, as they are also implicated in regulating parasite virulence. In undertaking their roles, heat shock proteins operate in networks that involve not only partners of parasite origin, but also potentially functionally associate with human proteins to facilitate parasite survival and pathogenicity. This review seeks to highlight these interplays and their roles in parasite pathogenicity. We further discuss the prospects of targeting the parasite heat shock protein network towards the developments of alternative antimalarial chemotherapies.
Keywords: Plasmodium falciparum; chaperone; co-chaperone; exportome; functional interplay; heat shock proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Bioprospecting for Novel Heat Shock Protein Modulators: The New Frontier for Antimalarial Drug Discovery?Adv Exp Med Biol. 2021;1340:187-203. doi: 10.1007/978-3-030-78397-6_8. Adv Exp Med Biol. 2021. PMID: 34569026
-
Heat Shock Proteins of Malaria: Highlights and Future Prospects.Adv Exp Med Biol. 2021;1340:237-246. doi: 10.1007/978-3-030-78397-6_10. Adv Exp Med Biol. 2021. PMID: 34569028
-
Plasmodium falciparum heat shock proteins as antimalarial drug targets: An update.Cell Stress Chaperones. 2024 Apr;29(2):326-337. doi: 10.1016/j.cstres.2024.03.007. Epub 2024 Mar 20. Cell Stress Chaperones. 2024. PMID: 38518861 Free PMC article. Review.
-
The Plasmodium falciparum Hsp70-x chaperone assists the heat stress response of the malaria parasite.FASEB J. 2019 Dec;33(12):14611-14624. doi: 10.1096/fj.201901741R. Epub 2019 Nov 14. FASEB J. 2019. PMID: 31690116 Free PMC article.
-
The Hsp40 proteins of Plasmodium falciparum and other apicomplexa: regulating chaperone power in the parasite and the host.Int J Biochem Cell Biol. 2007;39(10):1781-803. doi: 10.1016/j.biocel.2007.02.011. Epub 2007 Feb 22. Int J Biochem Cell Biol. 2007. PMID: 17428722 Review.
Cited by
-
The Link That Binds: The Linker of Hsp70 as a Helm of the Protein's Function.Biomolecules. 2019 Sep 27;9(10):543. doi: 10.3390/biom9100543. Biomolecules. 2019. PMID: 31569820 Free PMC article. Review.
-
Small Molecule Inhibitors Targeting the Heat Shock Protein System of Human Obligate Protozoan Parasites.Int J Mol Sci. 2019 Nov 25;20(23):5930. doi: 10.3390/ijms20235930. Int J Mol Sci. 2019. PMID: 31775392 Free PMC article. Review.
-
Unraveling the Role of Proteinopathies in Parasitic Infections.Biomedicines. 2025 Mar 3;13(3):610. doi: 10.3390/biomedicines13030610. Biomedicines. 2025. PMID: 40149586 Free PMC article. Review.
-
Plasmodium berghei Hsp90 contains a natural immunogenic I-Ab-restricted antigen common to rodent and human Plasmodium species.Curr Res Immunol. 2021 Jun 30;2:79-92. doi: 10.1016/j.crimmu.2021.06.002. eCollection 2021. Curr Res Immunol. 2021. PMID: 35492393 Free PMC article.
-
Violacein-Induced Chaperone System Collapse Underlies Multistage Antiplasmodial Activity.ACS Infect Dis. 2021 Apr 9;7(4):759-776. doi: 10.1021/acsinfecdis.0c00454. Epub 2021 Mar 10. ACS Infect Dis. 2021. PMID: 33689276 Free PMC article.
References
-
- World Health Organization . World Malaria Report 2018. World Health Organization; Geneva, Switzerland: 2018.
-
- Olshina M.A., Baumann H., Willison K.R., Baum J. Plasmodium actin is incompletely folded by heterologous protein-folding machinery and likely requires the native Plasmodium chaperonin complex to enter a mature functional state. FASEB J. 2016;30:405–416. doi: 10.1096/fj.15-276618. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

