Cell Death in the Kidney
- PMID: 31340541
- PMCID: PMC6679187
- DOI: 10.3390/ijms20143598
Cell Death in the Kidney
Abstract
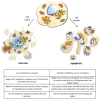
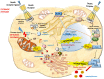
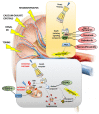
Apoptotic cell death is usually a response to the cell's microenvironment. In the kidney, apoptosis contributes to parenchymal cell loss in the course of acute and chronic renal injury, but does not trigger an inflammatory response. What distinguishes necrosis from apoptosis is the rupture of the plasma membrane, so necrotic cell death is accompanied by the release of unprocessed intracellular content, including cellular organelles, which are highly immunogenic proteins. The relative contribution of apoptosis and necrosis to injury varies, depending on the severity of the insult. Regulated cell death may result from immunologically silent apoptosis or from immunogenic necrosis. Recent advances have enhanced the most revolutionary concept of regulated necrosis. Several modalities of regulated necrosis have been described, such as necroptosis, ferroptosis, pyroptosis, and mitochondrial permeability transition-dependent regulated necrosis. We review the different modalities of apoptosis, necrosis, and regulated necrosis in kidney injury, focusing particularly on evidence implicating cell death in ectopic renal calcification. We also review the evidence for the role of cell death in kidney injury, which may pave the way for new therapeutic opportunities.
Keywords: apoptosis; glomerular injury; kidney injury; necrosis; regulated necrosis; tubular injury.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- Bessis M. Studies on cell agony and death: An attempt at classification. In: de Reuck A.V.S., Knight J., editors. Ciba Foundation Symposium - Cellular Injury. J&A Churchill; London, UK: 1964.
-
- Green D.R. Means to an End: Apoptosis and Other Cell Death Mechanisms. 1st ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2010.
-
- Galluzzi L., Bravo-San Pedro J.M., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Alnemri E.S., Altucci L., Andrews D., Annicchiarico-Petruzzelli M., et al. Essential versus accessory aspects of cell death: Recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

