Formation and Resolution of Pial Microvascular Thrombosis in a Mouse Model of Thrombotic Thrombocytopenic Purpura
- PMID: 31340669
- PMCID: PMC6709991
- DOI: 10.1161/ATVBAHA.119.312848
Formation and Resolution of Pial Microvascular Thrombosis in a Mouse Model of Thrombotic Thrombocytopenic Purpura
Abstract
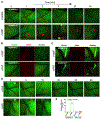
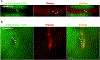
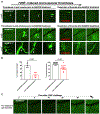
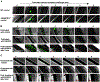
Objective: Microvascular thrombosis is the hallmark pathology of thrombotic thrombocytopenic purpura (TTP), a rare life-threatening disease. Neurological dysfunction is present in over 90% of patients with TTP, and TTP can cause long-lasting neurological damage or death. However, the pathophysiology of microvascular thrombosis in the brain is not well studied to date. Here, we investigate the formation and resolution of thrombosis in pial microvessels. Approach and Results: Using a cranial intravital microscopy in well-established mouse models of congenital TTP induced by infusion of recombinant VWF (von Willebrand factor), we found that soluble VWF, at high concentration, adheres to the endothelium of the vessel wall, self-associates, and initiates platelet adhesion resulting in the formation of pial microvascular thrombosis in ADAMTS13-/- (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) mice. Importantly, VWF-mediated pial microvascular thrombosis occurred without vascular injury to the brain, and thrombi consisted of resting platelets adhered onto ultra-large VWF without fibrin in the brain in rVWF (recombinant VWF) challenged ADAMTS13-/- mice. Prophylactic treatment with recombinant ADAMTS13 (BAX930) effectively prevented the onset of the VWF-mediated microvascular thrombosis and therapeutic treatment with BAX930 acutely resolved the preexisting or growing thrombi in the brain of ADAMTS13-/- mice after rVWF challenge. The absence of platelet activation and fibrin formation within VWF-mediated thrombi and efficacy of BAX930 was confirmed with an endothelial-driven VWF-mediated microvascular thrombosis model in mice.
Conclusions: Our results provide important insight into the initiation and development of microvascular thrombi in mouse models that mimics TTP and indicate that rADAMTS13 could be an effective interventional therapy for microvascular thrombosis, the hallmark pathology in TTP.
Keywords: blood platelets; fibrin; platelet aggregation; thrombosis; von Willebrand factor.
Conflict of interest statement
Disclosure of conflict of interest
Drs. Holinstat and Adili received financial support from Shire for the research presented here. Dr. Holinstat is a consultant and equity holder for Veralox Therapeutics, although no work presented here is associated with the relationship with Veralox Therapeutics. The authors declare no additional financial interests for the work presented here. The authors were solely responsible for the content and decision to publish.
Figures

Similar articles
-
Transfusion of Platelets Loaded With Recombinant ADAMTS13 (A Disintegrin and Metalloprotease With Thrombospondin Type 1 Repeats-13) Is Efficacious for Inhibiting Arterial Thrombosis Associated With Thrombotic Thrombocytopenic Purpura.Arterioscler Thromb Vasc Biol. 2018 Nov;38(11):2731-2743. doi: 10.1161/ATVBAHA.118.311407. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354235 Free PMC article.
-
Plasmin cleavage of von Willebrand factor as an emergency bypass for ADAMTS13 deficiency in thrombotic microangiopathy.Circulation. 2014 Mar 25;129(12):1320-31. doi: 10.1161/CIRCULATIONAHA.113.006727. Epub 2014 Jan 21. Circulation. 2014. PMID: 24449821
-
Preventative and therapeutic effects of a novel humanized anti-glycoprotein Ibα fragment of antigen-binding region in a murine model of thrombotic thrombocytopenic purpura.J Thromb Haemost. 2025 May;23(5):1596-1607. doi: 10.1016/j.jtha.2025.02.009. Epub 2025 Feb 15. J Thromb Haemost. 2025. PMID: 39956431
-
Thrombotic thrombocytopenic purpura: basic pathophysiology and therapeutic strategies.Hematology Am Soc Hematol Educ Program. 2013;2013:292-9. doi: 10.1182/asheducation-2013.1.292. Hematology Am Soc Hematol Educ Program. 2013. PMID: 24319194 Review.
-
Thrombosis and von Willebrand Factor.Adv Exp Med Biol. 2017;906:285-306. doi: 10.1007/5584_2016_122. Adv Exp Med Biol. 2017. PMID: 27628010 Review.
Cited by
-
Clot Accumulation in 3D Microfluidic Bifurcating Microvasculature Network.Micromachines (Basel). 2024 Jul 31;15(8):988. doi: 10.3390/mi15080988. Micromachines (Basel). 2024. PMID: 39203639 Free PMC article.
-
Targeting von Willebrand factor in liver diseases: A novel therapeutic strategy?J Thromb Haemost. 2021 Jun;19(6):1390-1408. doi: 10.1111/jth.15312. Epub 2021 May 3. J Thromb Haemost. 2021. PMID: 33774926 Free PMC article. Review.
-
Low-density lipoprotein promotes microvascular thrombosis by enhancing von Willebrand factor self-association.Blood. 2023 Sep 28;142(13):1156-1166. doi: 10.1182/blood.2023019749. Blood. 2023. PMID: 37506337 Free PMC article.
-
Recent Insights Into the Regulation of Coagulation and Thrombosis.Arterioscler Thromb Vasc Biol. 2020 May;40(5):e119-e125. doi: 10.1161/ATVBAHA.120.312674. Epub 2020 Apr 22. Arterioscler Thromb Vasc Biol. 2020. PMID: 32320291 Free PMC article. Review.
-
[Recurrent thrombocytopenia with hemolytic anemia in a boy aged 3 years].Zhongguo Dang Dai Er Ke Za Zhi. 2021 May;23(5):524-529. doi: 10.7499/j.issn.1008-8830.2101085. Zhongguo Dang Dai Er Ke Za Zhi. 2021. PMID: 34020745 Free PMC article. Chinese.
References
-
- Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program. 2004:407–423 - PubMed
-
- Kremer Hovinga JA, Coppo P, Lammle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3:17020. - PubMed
-
- Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD Jr., Ginsburg D, Tsai HM. Mutations in a member of the adamts gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494 - PubMed
-
- Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von willebrand factor-cleaving protease (adamts13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–41063 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

