The role of Activin A in fibrodysplasia ossificans progressiva: a prominent mediator
- PMID: 31341010
- PMCID: PMC6680371
- DOI: 10.1042/BSR20190377
The role of Activin A in fibrodysplasia ossificans progressiva: a prominent mediator
Abstract
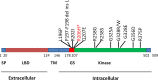
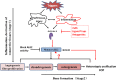
Heterotopic ossification (HO) is the aberrant formation of mature, lamellar bone in nonosseous tissue. Fibrodysplasia ossificans progressiva (FOP) is a rare and devastating genetic disorder that causes progressive HO in the ligaments, tendons, and muscles throughout the body. FOP is attributed to an autosomal mutation in activin receptor-like kinase 2 (ALK2), a bone morphogenetic protein (BMP) type I receptor. Initial studies show that mutant ALK2 drives HO by constitutively activating the BMP signaling pathway. Recently, mutant ALK2 has been shown to transduce Smad1/5 signaling and enhance chondrogenesis, calcification in response to Activin A, which normally signals through Smad2/3 and inhibits BMP signaling pathway. Furthermore, Activin A induces heterotopic bone formation via mutant ALK2, while inhibition of Activin A blocks spontaneous and trauma-induced HO. In this manuscript, we describe the molecular mechanism of the causative gene ALK2 in FOP, mainly focusing on the prominent role of Activin A in HO. It reveals a potential strategy for prevention and treatment of FOP by inhibition of Activin A. Further studies are needed to explore the cellular and molecular mechanisms of Activin A in FOP in more detail.
Keywords: ALK2; Activin A; FOP; Heterotopic ossification.
© 2019 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

