Remote sensing and signaling in kidney proximal tubules stimulates gut microbiome-derived organic anion secretion
- PMID: 31341083
- PMCID: PMC6689987
- DOI: 10.1073/pnas.1821809116
Remote sensing and signaling in kidney proximal tubules stimulates gut microbiome-derived organic anion secretion
Abstract
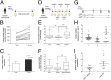
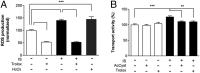
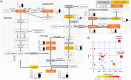
Membrane transporters and receptors are responsible for balancing nutrient and metabolite levels to aid body homeostasis. Here, we report that proximal tubule cells in kidneys sense elevated endogenous, gut microbiome-derived, metabolite levels through EGF receptors and downstream signaling to induce their secretion by up-regulating the organic anion transporter-1 (OAT1). Remote metabolite sensing and signaling was observed in kidneys from healthy volunteers and rats in vivo, leading to induced OAT1 expression and increased removal of indoxyl sulfate, a prototypical microbiome-derived metabolite and uremic toxin. Using 2D and 3D human proximal tubule cell models, we show that indoxyl sulfate induces OAT1 via AhR and EGFR signaling, controlled by miR-223. Concomitantly produced reactive oxygen species (ROS) control OAT1 activity and are balanced by the glutathione pathway, as confirmed by cellular metabolomic profiling. Collectively, we demonstrate remote metabolite sensing and signaling as an effective OAT1 regulation mechanism to maintain plasma metabolite levels by controlling their secretion.
Keywords: indoxyl sulfate; kidney proximal tubule; organic anion transporter 1; remote sensing and signaling.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

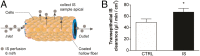
Comment in
-
Remote sensing and signalling in the kidney controls microbial metabolite levels.Nat Rev Nephrol. 2019 Oct;15(10):594. doi: 10.1038/s41581-019-0195-7. Nat Rev Nephrol. 2019. PMID: 31395969 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

