Functional importance of the oligomer formation of the cyanobacterial H+ pump Gloeobacter rhodopsin
- PMID: 31341208
- PMCID: PMC6656774
- DOI: 10.1038/s41598-019-47178-5
Functional importance of the oligomer formation of the cyanobacterial H+ pump Gloeobacter rhodopsin
Abstract
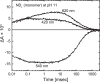
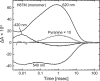
Many microbial rhodopsins self-oligomerize, but the functional consequences of oligomerization have not been well clarified. We examined the effects of oligomerization of a H+ pump, Gloeobacter rhodopsin (GR), by using nanodisc containing trimeric and monomeric GR. The monomerization did not appear to affect the unphotolyzed GR. However, we found a significant impact on the photoreaction: The monomeric GR showed faint M intermediate formation and negligible H+ transfer reactions. These changes reflected the elevated pKa of the Asp121 residue, whose deprotonation is a prerequisite for the functional photoreaction. Here, we focused on His87, which is a neighboring residue of Asp121 and conserved among eubacterial H+ pumps but replaced by Met in an archaeal H+ pump. We found that the H87M mutation removes the "monomerization effects": Even in the monomeric state, H87M contained the deprotonated Asp121 and showed both M formation and distinct H+ transfer reactions. Thus, for wild-type GR, monomerization probably strengthens the Asp121-His87 interaction and thereby elevates the pKa of Asp121 residue. This strong interaction might occur due to the loosened protein structure and/or the disruption of the interprotomer interaction of His87. Thus, the trimeric assembly of GR enables light-induced H+ transfer reactions through adjusting the positions of key residues.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Salt bridge in the conserved His-Asp cluster in Gloeobacter rhodopsin contributes to trimer formation.FEBS Lett. 2013 Feb 14;587(4):322-7. doi: 10.1016/j.febslet.2012.12.022. Epub 2013 Jan 9. FEBS Lett. 2013. PMID: 23313943
-
Cyanobacterial light-driven proton pump, gloeobacter rhodopsin: complementarity between rhodopsin-based energy production and photosynthesis.PLoS One. 2014 Oct 27;9(10):e110643. doi: 10.1371/journal.pone.0110643. eCollection 2014. PLoS One. 2014. PMID: 25347537 Free PMC article.
-
X-ray Crystallographic Structure and Oligomerization of Gloeobacter Rhodopsin.Sci Rep. 2019 Aug 2;9(1):11283. doi: 10.1038/s41598-019-47445-5. Sci Rep. 2019. PMID: 31375689 Free PMC article.
-
Comparative studies on ion pumps of the bacterial rhodopsin family.Biophys Chem. 1994 May;50(1-2):191-201. doi: 10.1016/0301-4622(94)85031-3. Biophys Chem. 1994. PMID: 8011934 Review.
-
Channelrhodopsin unchained: structure and mechanism of a light-gated cation channel.Biochim Biophys Acta. 2014 May;1837(5):626-42. doi: 10.1016/j.bbabio.2013.10.014. Epub 2013 Nov 7. Biochim Biophys Acta. 2014. PMID: 24212055 Review.
Cited by
-
Proteorhodopsin insights into the molecular mechanism of vectorial proton transport.Sci Adv. 2025 Apr 18;11(16):eadu5303. doi: 10.1126/sciadv.adu5303. Epub 2025 Apr 16. Sci Adv. 2025. PMID: 40238873 Free PMC article.
-
Retinal-Carotenoid Interactions in a Sodium-Ion-Pumping Rhodopsin: Implications on Oligomerization and Thermal Stability.J Phys Chem B. 2023 Mar 16;127(10):2128-2137. doi: 10.1021/acs.jpcb.2c07502. Epub 2023 Mar 1. J Phys Chem B. 2023. PMID: 36857147 Free PMC article.
-
Reisomerization of retinal represents a molecular switch mediating Na+ uptake and release by a bacterial sodium-pumping rhodopsin.J Biol Chem. 2022 Sep;298(9):102366. doi: 10.1016/j.jbc.2022.102366. Epub 2022 Aug 11. J Biol Chem. 2022. PMID: 35963435 Free PMC article.
-
Cyanorhodopsin-II represents a yellow-absorbing proton-pumping rhodopsin clade within cyanobacteria.ISME J. 2024 Jan 8;18(1):wrae175. doi: 10.1093/ismejo/wrae175. ISME J. 2024. PMID: 39485071 Free PMC article.
-
Mutations conferring SO42- pumping ability on the cyanobacterial anion pump rhodopsin and the resultant unique features of the mutant.Sci Rep. 2022 Sep 30;12(1):16422. doi: 10.1038/s41598-022-20784-6. Sci Rep. 2022. PMID: 36180556 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

