Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling
- PMID: 31341276
- PMCID: PMC6697195
- DOI: 10.1038/s41586-019-1426-6
Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling
Erratum in
-
Publisher Correction: Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling.Nature. 2019 Aug;572(7770):E20. doi: 10.1038/s41586-019-1480-0. Nature. 2019. PMID: 31371811
Abstract
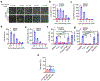
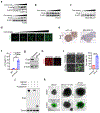
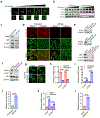
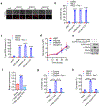
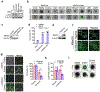
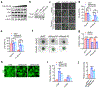
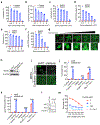
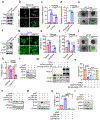
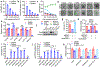
Ferroptosis, a cell death process driven by cellular metabolism and iron-dependent lipid peroxidation, has been implicated in diseases such as ischaemic organ damage and cancer1,2. The enzyme glutathione peroxidase 4 (GPX4) is a central regulator of ferroptosis, and protects cells by neutralizing lipid peroxides, which are by-products of cellular metabolism. The direct inhibition of GPX4, or indirect inhibition by depletion of its substrate glutathione or the building blocks of glutathione (such as cysteine), can trigger ferroptosis3. Ferroptosis contributes to the antitumour function of several tumour suppressors such as p53, BAP1 and fumarase4-7. Counterintuitively, mesenchymal cancer cells-which are prone to metastasis, and often resistant to various treatments-are highly susceptible to ferroptosis8,9. Here we show that ferroptosis can be regulated non-cell-autonomously by cadherin-mediated intercellular interactions. In epithelial cells, such interactions mediated by E-cadherin suppress ferroptosis by activating the intracellular NF2 (also known as merlin) and Hippo signalling pathway. Antagonizing this signalling axis allows the proto-oncogenic transcriptional co-activator YAP to promote ferroptosis by upregulating several ferroptosis modulators, including ACSL4 and TFRC. This finding provides mechanistic insights into the observations that cancer cells with mesenchymal or metastatic property are highly sensitive to ferroptosis8. Notably, a similar mechanism also modulates ferroptosis in some non-epithelial cells. Finally, genetic inactivation of the tumour suppressor NF2, a frequent tumorigenic event in mesothelioma10,11, rendered cancer cells more sensitive to ferroptosis in an orthotopic mouse model of malignant mesothelioma. Our results demonstrate the role of intercellular interactions and intracellular NF2-YAP signalling in dictating ferroptotic death, and also suggest that malignant mutations in NF2-YAP signalling could predict the responsiveness of cancer cells to future ferroptosis-inducing therapies.
Figures


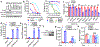
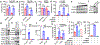

Comment in
-
Cancer-cell death ironed out.Nature. 2019 Aug;572(7769):314-315. doi: 10.1038/d41586-019-02218-y. Nature. 2019. PMID: 31406306 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

