Relationships between plant traits, soil properties and carbon fluxes differ between monocultures and mixed communities in temperate grassland
- PMID: 31341333
- PMCID: PMC6617750
- DOI: 10.1111/1365-2745.13160
Relationships between plant traits, soil properties and carbon fluxes differ between monocultures and mixed communities in temperate grassland
Abstract
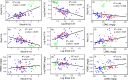
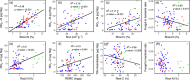
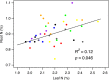
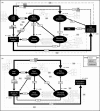
The use of plant traits to predict ecosystem functions has been gaining growing attention. Above-ground plant traits, such as leaf nitrogen (N) content and specific leaf area (SLA), have been shown to strongly relate to ecosystem productivity, respiration and nutrient cycling. Furthermore, increasing plant functional trait diversity has been suggested as a possible mechanism to increase ecosystem carbon (C) storage. However, it is uncertain whether below-ground plant traits can be predicted by above-ground traits, and if both above- and below-ground traits can be used to predict soil properties and ecosystem-level functions.Here, we used two adjacent field experiments in temperate grassland to investigate if above- and below-ground plant traits are related, and whether relationships between plant traits, soil properties and ecosystem C fluxes (i.e. ecosystem respiration and net ecosystem exchange) measured in potted monocultures could be detected in mixed field communities.We found that certain shoot traits (e.g. shoot N and C, and leaf dry matter content) were related to root traits (e.g. root N, root C:N and root dry matter content) in monocultures, but such relationships were either weak or not detected in mixed communities. Some relationships between plant traits (i.e. shoot N, root N and/or shoot C:N) and soil properties (i.e. inorganic N availability and microbial community structure) were similar in monocultures and mixed communities, but they were more strongly linked to shoot traits in monocultures and root traits in mixed communities. Structural equation modelling showed that above- and below-ground traits and soil properties improved predictions of ecosystem C fluxes in monocultures, but not in mixed communities on the basis of community-weighted mean traits. Synthesis. Our results from a single grassland habitat detected relationships in monocultures between above- and below-ground plant traits, and between plant traits, soil properties and ecosystem C fluxes. However, these relationships were generally weaker or different in mixed communities. Our results demonstrate that while plant traits can be used to predict certain soil properties and ecosystem functions in monocultures, they are less effective for predicting how changes in plant species composition influence ecosystem functions in mixed communities.
Keywords: above‐ground–below‐ground linkages; biodiversity; carbon; ecosystem function; net ecosystem exchange; nitrogen; plant functional traits; soil microbial communities.
Figures


References
-
- Albert, C. H. , Grassein, F. , Schurr, F. M. , Vieilledent, G. , & Violle, C. (2011). When and how should intraspecific variability be considered in trait‐based plant ecology? Perspectives in Plant Ecology Evolution and Systematics, 13, 217–225.
-
- Bardgett, R. D. (2017). Plant trait‐based approaches for interrogating belowground function. Biology and Environment . Proceedings of the Royal Irish Academy, 117B, 1–13.
-
- Bardgett, R. D. , & McAlister, E. (1999). The measurement of soil fungal: Bacterial biomass ratios as an indicator of ecosystem self‐regulation in temperate meadow grasslands. Biology and Fertility of Soils, 29, 282–290. 10.1007/s003740050554 - DOI
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
