The characterisation of pulmonary function in patients with mucopolysaccharidoses IVA: A longitudinal analysis
- PMID: 31341787
- PMCID: PMC6629586
- DOI: 10.1016/j.ymgmr.2019.100487
The characterisation of pulmonary function in patients with mucopolysaccharidoses IVA: A longitudinal analysis
Abstract
Introduction: Mucopolysaccharidosis (MPS) type IVA is a rare, autosomal recessive lysosomal storage disease causing substrate accumulation in various organs and tissues. MPS IVA is associated with both obstructive and restrictive airway disease, with the former often resulting in sleep disordered breathing (SDB). Respiratory failure is a primary cause of death in this condition. The aim of this study was to characterise and catalogue the long-term respiratory changes in patients with MPS IVA treated with, or without, enzyme replacement therapy (ERT).
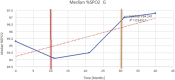
Methods: In this retrospective, longitudinal, repeated-measures cohort study, descriptive statistics and non-parametric correlation were performed for demographic, respiratory function and oximetry variables over a study period from January 2009 to December 2018. Composite clinical endpoints used in this study for evaluating pulmonary function included spirometry variables (FEV1, FEV1 [%Pred] FVC, FVC [%Pred] and FEV1/FVC), oximetry variables (median %Spo2, ODI 3%, mean nadir 3%, ODI 4%, mean nadir 4% and min dip SpO2 [%]) and 6MWT to assess functional exercise capacity and thus integrated cardiopulmonary function.
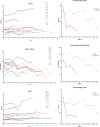
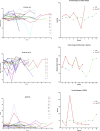
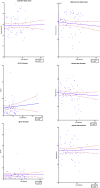
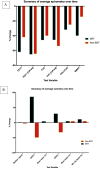
Results: Sequential spirometry and oximetry values were collected from 16 patients, of which 13/16 were ERT treated. In general, during the study period there was a global reduction in static spirometry values in all subjects, as well as cardiorespiratory function as assessed by the 6MWT, with the decline being delayed in the ERT group. Oximetry changed to a minor degree over time in the ERT group, whereas it declined in the non-ERT group. FEV1, FVC [%predicted] and ODI 3% exhibited a strong, combined positive correlation (r 0.74-95% CI 0.61 to 0.83; p < .0001). Non-invasive ventilation (NIV) and adenotonsillectomy appeared more effective in the ERT group, either improving pulmonary function or attenuating deterioration.
Conclusions: Whilst spirometry values showed a gradual decline across all groups, oximetry showed modest improvement in respiratory function. The amalgamation of FEV1, FVC [%predicted] and ODI 3% appeared predictive of changes in respiratory function in this study, suggestive as being composite endpoints for monitoring disease progression as well as guiding response to ERT in MPS IVA patients.
Keywords: 6MWT, 6-minute walk test; AASM, American Academy of Sleep Medicine; ADLs, Activities of daily living; AEs, Adverse Events; ATS, American Thoracic Society; BTS, British Thoracic Society; BiPAP, Bi-level non-invasive ventilation; C6S, Chondroitin Sulphate; CPET, Cardiopulmonary exercises testing; ECM, Extracellular matrix; ERT, Enzyme replacement therapy; Enzyme replacement therapy; FDA, Food and Drug Administration; FEV1 [%Pred], FEV1 as a percentage of predicted; FEV1, Forced expiratory volume in one second; FVC, Forced vital capacity; FVC: [%Pred], FVC as a percentage of predicted; GAG, Glycosaminoglycan; GALNS, Acetylgalactosamine-6-sulfatase; KS, Keratan sulfate; LSD, Lysosomal storage disease.; MPS; MPS IVA, Mucopolysaccharidosis Type IVA; MPS, Mucopolysaccharidosis; Med nadir 3%, Median nadir of arterial oxygen saturations 3% from baseline; Min dip Spo2, Minimum dips in arterial oxygen saturations [%]; Morquio syndrome; Mucopolysaccharidosis IVA; ODI 3%, Oxygen desaturation index; ≥ 3% arterial oxygen desaturations per hour; OSA, Obstructive Sleep Apnea; Respiratory changes; Sleep disordered breathing; Spo2, Arterial saturations; T&A, Adenotonsillectomy; uKS, Urinary keratan sulfate.
Figures



References
-
- Morquio L. Sur une forme de dystrophie osseuse familiale. Bull. Soc. Pediatr. Paris. 1929;27:145–152.
-
- Jones S., Wynn R. Mucopolysaccharidoses: Clinical Features and Diagnosis – UpToDate. 2019. https://www.uptodate.com/contents/mucopolysaccharidoses-clinical-feature...
-
- Jones S., Wijburg F. Inborn Metab. Dis. Diagn. Treat. 6th ed. Springer; Berlin: 2016. Mucopolysaccharidoses, oligosaccharidoses and sialic acid disorders; p. 577.
-
- Tomatsu S., Montaño A.M., Oikawa H., Smith M., Barrera L., Chinen Y., Thacker M.M., Mackenzie W.G., Suzuki Y., Orii T. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr. Pharm. Biotechnol. 2011;12:931–945. - PubMed
LinkOut - more resources
Full Text Sources

