Sex differences in reward- and punishment-guided actions
- PMID: 31342271
- PMCID: PMC6861363
- DOI: 10.3758/s13415-019-00736-w
Sex differences in reward- and punishment-guided actions
Abstract
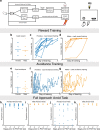
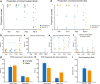
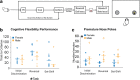
Differences in the prevalence and presentation of psychiatric illnesses in men and women suggest that neurobiological sex differences confer vulnerability or resilience in these disorders. Rodent behavioral models are critical for understanding the mechanisms of these differences. Reward processing and punishment avoidance are fundamental dimensions of the symptoms of psychiatric disorders. Here we explored sex differences along these dimensions using multiple and distinct behavioral paradigms. We found no sex difference in reward-guided associative learning but a faster punishment-avoidance learning in females. After learning, females were more sensitive than males to probabilistic punishment but less sensitive when punishment could be avoided with certainty. No sex differences were found in reward-guided cognitive flexibility. Thus, sex differences in goal-directed behaviors emerged selectively when there was an aversive context. These differences were critically sensitive to whether the punishment was certain or unpredictable. Our findings with these new paradigms provide conceptual and practical tools for investigating brain mechanisms that account for sex differences in susceptibility to anxiety and impulsivity. They may also provide insight for understanding the evolution of sex-specific optimal behavioral strategies in dynamic environments.
Keywords: ADHD; Addiction; Anxiety; Avoidance; Reward; Sex differences.
Figures

References
-
- Abuse, S. (2013). Mental Health Services Administration,(SAMHSA), 2013 Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No.(SMA) 13-4795. Substance Abuse and Mental Health Services Administration, Rockville.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

