Abatacept in Early Diffuse Cutaneous Systemic Sclerosis: Results of a Phase II Investigator-Initiated, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial
- PMID: 31342624
- PMCID: PMC6935399
- DOI: 10.1002/art.41055
Abatacept in Early Diffuse Cutaneous Systemic Sclerosis: Results of a Phase II Investigator-Initiated, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial
Abstract
Objective: T cells play a key role in the pathogenesis of early systemic sclerosis. This study was undertaken to assess the safety and efficacy of abatacept in patients with diffuse cutaneous systemic sclerosis (dcSSc).
Methods: In this 12-month, randomized, double-blind, placebo-controlled trial, participants were randomized 1:1 to receive either subcutaneous abatacept 125 mg or matching placebo, stratified by duration of dcSSc. Escape therapy was allowed at 6 months for worsening disease. The coprimary end points were change in the modified Rodnan skin thickness score (MRSS) compared to baseline and safety over 12 months. Differences in longitudinal outcomes were assessed according to treatment using linear mixed models, with outcomes censored after initiation of escape therapy. Skin tissue obtained from participants at baseline was classified into intrinsic gene expression subsets.
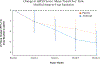
Results: Among 88 participants, the adjusted mean change in the MRSS at 12 months was -6.24 units for those receiving abatacept and -4.49 units for those receiving placebo, with an adjusted mean treatment difference of -1.75 units (P = 0.28). Outcomes for 2 secondary measures (Health Assessment Questionnaire disability index and a composite measure) were clinically and statistically significantly better with abatacept. The proportion of subjects in whom escape therapy was needed was higher in the placebo group relative to the abatacept group (36% versus 16%). In the inflammatory and normal-like skin gene expression subsets, decline in the MRSS over 12 months was clinically and significantly greater in the abatacept group versus the placebo group (P < 0.001 and P = 0.03, respectively). In the abatacept group, adverse events occurred in 35 participants versus 40 participants in the placebo group, including 2 deaths and 1 death, respectively.
Conclusion: In this phase II trial, abatacept was well-tolerated, but change in the MRSS was not statistically significant. Secondary outcome measures, including gene expression subsets, showed evidence in support of abatacept. These data should be confirmed in a phase III trial.
Trial registration: ClinicalTrials.gov NCT02161406.
© 2019, American College of Rheumatology.
Conflict of interest statement
Conflicts of interest:
Dr Khanna is a consultant to Actelion, Acceleron, Arena, Bayer, Boehringer Ingelheim, Bristol-Myer Squibb, CSL Behring, Corbus, Cytori, GSK, Genentech/Roche, Galapagos, Merck, Mitsubishi Tanabi, and UCB; he has received grants as part of investigator-initiated trials (to the University of Michigan) from Bayer, Bristol-Myer Squibb, and Pfizer; and has stock options in Eicos Sciences, Inc and employment with Civi BioPharma, Inc. Dr. Chung is a consultant to Reata, Bristol-Myer Squibb, Boehringer Ingelheim, Mitsubishi Tanabe, Eicos Sciences, Inc. Dr. Chung has also received grants from United Therapeutics. Dr. Molitor is a consultant to Boehringer-Ingelheim and Amgen. He has also received grants from Pfizer. Dr. Lafyatis is a consultant to PRISM Biolab, Merck, Bristol Myers Squibb, Biocon, UCB, Formation, Sanofi, and Genentech/Roche. He has received grants from PRISM Biolab, Regeneron, Elpidera, and Kiniksa. Dr. Domsic is a consultant to Eicos Sciences Inc. and Boehringer-Ingelheim. Dr. Pope is a consultant for Actelion, Baxter, Bristol-Myer Squibb, Merck, and Roche. She has received research grants from Baxter, Bristol-Myer Squibb, Eicos Sciences Inc, Merck, Roche, and Seattle Genetics. Dr. Gordon has received grants from Corbus and Cumberland Pharmaceuticals. Dr. Schiopu has received research grants from Bristol-Myer Squibb and Bayer. Dr. Bernstein is a consultant to Genentech as a medical monitor for SLS III. She has also received grants for investigator-initiated research from Pfizer and ASPIRE Rheumatology program. Dr. Castelino is a consultant to Genentech and Boehringer. Dr. Allanore is a consultant to BMS, Genentech/Roche, Sanofi, Bayer, Actelion, and Inventiva. Dr. Allanore has also received grants from Genentech/Roche, Sanofi, and Inventiva. Dr. Matucci-Cerinic is a consultant and has received grants from Bristol-Myer Squibb. Dr. Fox is a consultant as a grant reviewer for Pfizer. He has also received research grants from Regeneron, Gilead, and Seattle Genetics. Drs. Spino, Johnson, Whitfield, Berrocal, Franks, Mehta, Steen, Simms, Gill, Kafaja, Frech, Hsu, Mayes, Young, Sandorfi, Park, Hant, Chatterjee, Ajam, Wang, Wood, Distler, Singer, Bush, and Furst report no conflict of interest pertinent to this manuscript.
Figures

References
-
- Denton CP, Khanna D: Systemic sclerosis. Lancet 2017, 390(10103):1685–1699. - PubMed
-
- Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, Distler O, Clements P, Cutolo M, Czirjak L, Damjanov N, Del Galdo F, Denton CP, Distler JHW, Foeldvari I, Figelstone K, Frerix M, Furst DE, Guiducci S, Hunzelmann N, Khanna D, Matucci-Cerinic M, Herrick AL, van den Hoogen F, van Laar JM, Riemekasten G, Silver R, Smith V, Sulli A, Tarner I et al. : Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017, 76(8):1327–1339. - PubMed
-
- Roumm AD, Whiteside TL, Medsger TA Jr., Rodnan GP: Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum 1984, 27(6):645–653. - PubMed
-
- Fleischmajer R, Perlish JS, Reeves JR: Cellular infiltrates in scleroderma skin. Arthritis Rheum 1977, 20(4):975–984. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

