Dialing in on pharmacological features for a therapeutic antioxidant small molecule
- PMID: 31342985
- PMCID: PMC6863055
- DOI: 10.1039/c9dt01800j
Dialing in on pharmacological features for a therapeutic antioxidant small molecule
Abstract
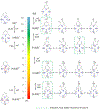
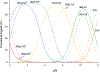
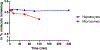
The pyridinophane molecule L2 (3,6,9,15-tetraazabicyclo[9.3.1]penta-deca-1(15),11,13-trien-13-ol) has shown promise as a therapuetic for neurodegenerative diseases involving oxidative stress and metal ion misregulation. Protonation and metal binding stability constants with Mg2+, Ca2+, Cu2+, and Zn2+ ions were determined to further explore the therapeutic and pharmacological potential of this water soluble small molecule. These studies show that incorporation of an -OH group in position 4 of the pyridine ring decreases the pI values compared to cyclen and L1 (3,6,9,15-tetraazabicyclo[9.3.1]penta-deca-1(15),11,13-triene). Furthermore, this approach tunes the basicity of the tetra-aza macrocyclic ligand through the enhanced resonance stabilization of the -OH in position 4 and rigidity of the pyridine ring such that L2 has increased basicity compared to previously reported tetra-aza macrocycles. A metal binding preference for Cu2+, a redox cycling agent known to produce oxidative stress, indicates that this would be the in vivo metal target of L2. However, the binding constant of L2 with Cu2+ is moderated compared to cyclen due to the rigidity of the ligand and shows how ligand design can be used to tune metal selectivity. An IC50 = 298.0 μM in HT-22 neuronal cells was observed. Low metabolic liability was determined in both Phase I and II in vitro models. Throughout these studies other metal binding systems were used for comparison and as appropriate controls. The reactivity reported to date and pharmacological features described herein warrant further studies in vivo and the pursuit of L2 congeners using the knowledge that pyridine substitution in a pyridinophane can be used to tune the structure of the ligand and retain the positive therapeutic outcomes.
Figures


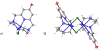

Similar articles
-
Structural, spectral, and electrochemical properties of nickel(II), copper(II), and zinc(II) complexes containing 12-membered pyridine- and pyridol-based tetra-aza macrocycles.Inorg Chem. 2014 Feb 3;53(3):1406-16. doi: 10.1021/ic402119s. Epub 2014 Jan 17. Inorg Chem. 2014. PMID: 24437677
-
Synthesis, Characterization, and Activity of a Triazine Bridged Antioxidant Small Molecule.ACS Chem Neurosci. 2017 Nov 15;8(11):2414-2423. doi: 10.1021/acschemneuro.7b00184. Epub 2017 Aug 22. ACS Chem Neurosci. 2017. PMID: 28768410
-
Anticancer activity of a cis-dichloridoplatinum(ii) complex of a chelating nitrogen mustard: insight into unusual guanine binding mode and low deactivation by glutathione.Dalton Trans. 2016 Feb 28;45(8):3599-615. doi: 10.1039/c5dt04459f. Dalton Trans. 2016. PMID: 26810988
-
Mechanistic Insight into the Design of Chemical Tools to Control Multiple Pathogenic Features in Alzheimer's Disease.Acc Chem Res. 2021 Oct 19;54(20):3930-3940. doi: 10.1021/acs.accounts.1c00457. Epub 2021 Oct 4. Acc Chem Res. 2021. PMID: 34606227 Review.
-
Small Molecule Catalysts with Therapeutic Potential.Molecules. 2018 Mar 27;23(4):765. doi: 10.3390/molecules23040765. Molecules. 2018. PMID: 29584669 Free PMC article. Review.
Cited by
-
Assembly of Polyiodide Networks with Cu(II) Complexes of Pyridinol-Based Tetraaza Macrocycles.Inorg Chem. 2022 Jan 10;61(1):368-383. doi: 10.1021/acs.inorgchem.1c02967. Epub 2021 Dec 21. Inorg Chem. 2022. PMID: 34933551 Free PMC article.
-
Hydrogen Peroxide Disproportionation with Manganese Macrocyclic Complexes of Cyclen and Pyclen.Inorg Chem Front. 2020 Apr 7;7(7):1573-1582. doi: 10.1039/c9qi01509d. Epub 2020 Mar 3. Inorg Chem Front. 2020. PMID: 32457818 Free PMC article.
-
Rings of Power: Controlling SOD Mimic Activity by the Addition of Pyridine Rings within the Pyridinophane Scaffold.Inorg Chem. 2024 Dec 16;63(50):23544-23553. doi: 10.1021/acs.inorgchem.4c02776. Epub 2024 Dec 3. Inorg Chem. 2024. PMID: 39625457
-
Pentadentate and Hexadentate Pyridinophane Ligands Support Reversible Cu(II)/Cu(I) Redox Couples.Inorganics (Basel). 2023 Nov;11(11):446. doi: 10.3390/inorganics11110446. Epub 2023 Nov 20. Inorganics (Basel). 2023. PMID: 39301085 Free PMC article.
-
A macrocyclic molecule with multiple antioxidative activities protects the lens from oxidative damage.Front Chem. 2022 Oct 28;10:996604. doi: 10.3389/fchem.2022.996604. eCollection 2022. Front Chem. 2022. PMID: 36385982 Free PMC article.
References
-
- Stetter H, Frank W and Mertens R, Tetrahedron, 1981, 37, 767–772.
-
- Alcock NW, Busch DH and Liu CY, Journal, 2007.
-
- Lincoln KM, Arroyo-Curras N, Johnston HM, Hayden TD, Pierce BS, Bhuvanesh N and Green KN, J Coord Chem, 2015, 68, 2810–2826.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

