Insight into D6h Symmetry: Targeting Strong Axiality in Stable Dysprosium(III) Hexagonal Bipyramidal Single-Ion Magnets
- PMID: 31343095
- PMCID: PMC6790654
- DOI: 10.1002/anie.201907686
Insight into D6h Symmetry: Targeting Strong Axiality in Stable Dysprosium(III) Hexagonal Bipyramidal Single-Ion Magnets
Abstract
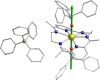
Following a novel synthetic strategy where the strong uniaxial ligand field generated by the Ph3 SiO- (Ph3 SiO- =anion of triphenylsilanol) and the 2,4-di-t Bu-PhO- (2,4-di-t Bu-PhO- =anion of 2,4-di-tertbutylphenol) ligands combined with the weak equatorial field of the ligand LN6 , leads to [DyIII (LN6 )(2,4-di-t Bu-PhO)2 ](PF6 ) (1), [DyIII (LN6 )(Ph3 SiO)2 ](PF6 ) (2) and [DyIII (LN6 )(Ph3 SiO)2 ](BPh4 ) (3) hexagonal bipyramidal dysprosium(III) single-molecule magnets (SMMs) with high anisotropy barriers of Ueff =973 K for 1, Ueff =1080 K for 2 and Ueff =1124 K for 3 under zero applied dc field. Ab initio calculations predict that the dominant magnetization reversal barrier of these complexes expands up to the 3rd Kramers doublet, thus revealing for the first time the exceptional uniaxial magnetic anisotropy that even the six equatorial donor atoms fail to negate, opening up the possibility to other higher-order symmetry SMMs.
Keywords: ab initio calculations; dysprosium; hexagonal bipyramid; magnetic properties; single molecule magnets.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- None
-
- Aubin S. M. J., Wemple M. W., Adams D. M., Tsai H.-L., Christou G., Hendrickson D. N., J. Am. Chem. Soc. 1996, 118, 7746–7754;
-
- Caneschi A., Gatteschi D., Sessoli R., Novak M. A., Nature 1993, 365, 141–143;
-
- Sessoli R., Tsai H. L., Schake A. R., Wang S., Vincent J. B., Folting K., Gatteschi D., Christou G., Hendrickson D. N., J. Am. Chem. Soc. 1993, 115, 1804–1816.
-
- None
Grants and funding
LinkOut - more resources
Full Text Sources

