Exosomes containing HIV protein Nef reorganize lipid rafts potentiating inflammatory response in bystander cells
- PMID: 31344124
- PMCID: PMC6657916
- DOI: 10.1371/journal.ppat.1007907
Exosomes containing HIV protein Nef reorganize lipid rafts potentiating inflammatory response in bystander cells
Abstract
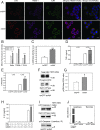
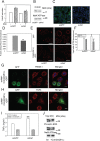
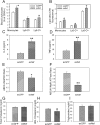
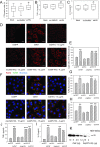
HIV infection has a profound effect on "bystander" cells causing metabolic co-morbidities. This may be mediated by exosomes secreted by HIV-infected cells and containing viral factors. Here we show that exosomes containing HIV-1 protein Nef (exNef) are rapidly taken up by macrophages releasing Nef into the cell interior. This caused down-regulation of ABCA1, reduction of cholesterol efflux and sharp elevation of the abundance of lipid rafts through reduced activation of small GTPase Cdc42 and decreased actin polymerization. Changes in rafts led to re-localization of TLR4 and TREM-1 to rafts, phosphorylation of ERK1/2, activation of NLRP3 inflammasome, and increased secretion of pro-inflammatory cytokines. The effects of exNef on lipid rafts and on inflammation were reversed by overexpression of a constitutively active mutant of Cdc42. Similar effects were observed in macrophages treated with exosomes produced by HIV-infected cells or isolated from plasma of HIV-infected subjects, but not with exosomes from cells and subjects infected with ΔNef-HIV or uninfected subjects. Mice injected with exNef exhibited monocytosis, reduced ABCA1 in macrophages, increased raft abundance in monocytes and augmented inflammation. Thus, Nef-containing exosomes potentiated pro-inflammatory response by inducing changes in cholesterol metabolism and reorganizing lipid rafts. These mechanisms may contribute to HIV-associated metabolic co-morbidities.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
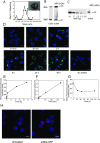
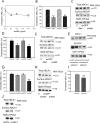
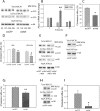
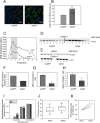

References
-
- Peters B, Post F, Wierzbicki AS, Phillips A, Power L, Das S, et al. Screening for chronic comorbid diseases in people with HIV: the need for a strategic approach. HIV Med. 2013;14:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

