Dexmedetomidine-induced cardioprotection is mediated by inhibition of high mobility group box-1 and the cholinergic anti-inflammatory pathway in myocardial ischemia-reperfusion injury
- PMID: 31344138
- PMCID: PMC6657822
- DOI: 10.1371/journal.pone.0218726
Dexmedetomidine-induced cardioprotection is mediated by inhibition of high mobility group box-1 and the cholinergic anti-inflammatory pathway in myocardial ischemia-reperfusion injury
Abstract
Objectives: Dexmedetomidine (DEX) is a selective α2-adrenoceptor agonist that has anti-inflammatory and cardioprotective effects in myocardial ischemia/reperfusion (I/R) injury. The present study aimed to investigate the underlying mechanism by which DEX protects against myocardial I/R.
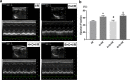
Methods: Sprague Dawley rats were subjected to either sham operation or myocardial I/R, which was induced by ligating the left anterior descending coronary artery for 30 min followed by reperfusion for 120 min. Rats were treated with either DEX or saline prior to surgery. We measured heart infarct size, serum cardiac Troponin I (cTnI), cardiac High mobility group box-1 (HMGB1) expression, myocardial apoptosis and cytokine production of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). Besides, we evaluated the heart function at 4 weeks post-reperfusion by echocardiography. Unilateral vagotomy or inhibition of the α7 nicotinic acetylcholine receptor (α7nAChR) with methyllycaconitine (MLA) was applied to investigate whether DEX-induced cardioprotection is mediated via the cholinergic anti-inflammatory pathway. Cardiac-selective overexpression of HMGB1 was administered to further confirm if HMGB1 is a key anti-inflammatory target during DEX-induced cardioprotection.
Results: DEX pretreatment significantly attenuated I/R-induced cardiac damage, as evidenced by decreases in short-term injury indicators including myocardial infarct size, cTnI release, myocardial apoptosis, cardiac HMGB1 expression, IL-6 and TNF-α production, as well as improvement on long-term cardiac function at 4 weeks post-reperfusion. These effects were partially reversed by either unilateral vagotomy or methyllycaconitine treatment. Besides, cardiac HMGB1-overexpression nearly abolished DEX-induced cardioprotection.
Conclusions: DEX pretreatment protects against myocardial I/R by inhibiting cardiac HMGB1 production and activating the cholinergic anti-inflammatory pathway.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures









References
-
- Ma A, Qi S, Chen H. Antioxidant therapy for prevention of inflammation, ischemic reperfusion injuries and allograft rejection. Cardiovasc Hematol Agents Med Chem. 2008;6(1):20–43. - PubMed
-
- Wang Z, Yu L, Wang S, Huang B, Liao K, Saren G, et al. Chronic intermittent low-level transcutaneous electrical stimulation of auricular branch of vagus nerve improves left ventricular remodeling in conscious dogs with healed myocardial infarction. Circ Heart Fail. 2014;7(6):1014–21. 10.1161/CIRCHEARTFAILURE.114.001564 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

