A novel defective recombinant porcine enterovirus G virus carrying a porcine torovirus papain-like cysteine protease gene and a putative anti-apoptosis gene in place of viral structural protein genes
- PMID: 31344488
- PMCID: PMC7105976
- DOI: 10.1016/j.meegid.2019.103975
A novel defective recombinant porcine enterovirus G virus carrying a porcine torovirus papain-like cysteine protease gene and a putative anti-apoptosis gene in place of viral structural protein genes
Abstract
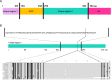
Enterovirus G (EV-G) belongs to the family of Picornaviridae. Two types of recombinant porcine EV-Gs carrying papain-like cysteine protease (PLCP) gene of porcine torovirus, a virus in Coronaviridae, are reported. Type 1 recombinant EV-Gs are detected in pig feces in Japan, USA, and Belgium and carry the PLPC gene at the junction site of 2C/3A genes, while PLPC gene replaces the viral structural genes in type 2 recombinant EV-G detected in pig feces in a Chinese farm. We identified a novel type 2 recombinant EV-G carrying the PLCP gene with flanking sequences in place of the viral structural genes in pig feces in Japan. The ~0.3 kb-long upstream flanking sequence had no sequence homology with any proteins deposited in GenBank, while the downstream ~0.9 kb-long flanking sequence included a domain having high amino acid sequence homology with a baculoviral inhibitor of apoptosis repeat superfamily. The pig feces, where the novel type 2 recombinant EV-G was detected, also carried type 1 recombinant EV-G. The amount of type 1 and type 2 recombinant EV-G genomes was almost same in the pig feces. Although the phylogenetic analysis suggested that these two recombinant EV-Gs have independently evolved, type 1 recombinant EV-G might have served as a helper virus by providing viral structural proteins for dissemination of the type 2 recombinant EV-G.
Keywords: Enterovirus; Recombination; Torovirus.
Copyright © 2019. Published by Elsevier B.V.
Figures








Similar articles
-
Novel recombinant porcine enterovirus G viruses lacking structural proteins are maintained in pig farms in Japan.J Vet Med Sci. 2023 Feb 21;85(2):252-265. doi: 10.1292/jvms.22-0505. Epub 2022 Dec 22. J Vet Med Sci. 2023. PMID: 36543238 Free PMC article.
-
First detection of novel enterovirus G recombining a torovirus papain-like protease gene associated with diarrhoea in swine in South Korea.Transbound Emerg Dis. 2019 Mar;66(2):1023-1028. doi: 10.1111/tbed.13073. Epub 2018 Dec 1. Transbound Emerg Dis. 2019. PMID: 30431236 Free PMC article.
-
Full-length and defective enterovirus G genomes with distinct torovirus protease insertions are highly prevalent on a Chinese pig farm.Arch Virol. 2018 Sep;163(9):2471-2476. doi: 10.1007/s00705-018-3875-x. Epub 2018 May 21. Arch Virol. 2018. PMID: 29786119
-
Genetic diversity of enterovirus G detected in faecal samples of wild boars in Japan: identification of novel genotypes carrying a papain-like cysteine protease sequence.J Gen Virol. 2020 Aug;101(8):840-852. doi: 10.1099/jgv.0.001446. Epub 2020 Jun 16. J Gen Virol. 2020. PMID: 32553066
-
Multiple genotypes of enterovirus G carrying a papain-like cysteine protease (PL-CP) sequence circulating on two pig farms in Japan: first identification of enterovirus G10 carrying a PL-CP sequence.Arch Virol. 2020 Dec;165(12):2909-2914. doi: 10.1007/s00705-020-04816-y. Epub 2020 Sep 19. Arch Virol. 2020. PMID: 32951133
Cited by
-
Colorimetric reverse transcription loop-mediated isothermal amplification assay for visual, sensitive, and specific detection of enterovirus G.BMC Vet Res. 2025 Aug 29;21(1):529. doi: 10.1186/s12917-025-04970-y. BMC Vet Res. 2025. PMID: 40883771 Free PMC article.
-
Novel recombinant porcine enterovirus G viruses lacking structural proteins are maintained in pig farms in Japan.J Vet Med Sci. 2023 Feb 21;85(2):252-265. doi: 10.1292/jvms.22-0505. Epub 2022 Dec 22. J Vet Med Sci. 2023. PMID: 36543238 Free PMC article.
-
Distribution and genetic diversity of Enterovirus G (EV-G) on pig farms in Thailand.BMC Vet Res. 2021 Aug 16;17(1):277. doi: 10.1186/s12917-021-02988-6. BMC Vet Res. 2021. PMID: 34399753 Free PMC article.
-
Reverse Genetics with a Full-Length Infectious cDNA Clone of Bovine Torovirus.J Virol. 2022 Feb 9;96(3):e0156121. doi: 10.1128/JVI.01561-21. Epub 2021 Nov 24. J Virol. 2022. PMID: 34817201 Free PMC article.
-
Construction of enterovirus G expressing reporter genes for antiviral drug screening assays.BMC Vet Res. 2025 Aug 13;21(1):515. doi: 10.1186/s12917-025-04960-0. BMC Vet Res. 2025. PMID: 40804379 Free PMC article.
References
-
- Bunke J., Receveur K., Oeser A.C., Fickenscher H., Zell R., Krumbholz A. High genetic diversity of porcine diversity of porcine enterovirus G in Schleswig-Holstein, Germany. Arch. Virol. 2018;163:489–493. - PubMed
-
- Conceição-Neto N., Theuns S., Cui T., Zeller M., Yinda C.K., Christiaens I., Heylen E., Van Ranst M., Carpentier S., Nauwynck H.J., Matthijnssens J. Identification of an enterovirus recombinant with a torovirus-like gene insertion during a diarrhea outbreak in fattening pigs. Virus Evol. 2017;3 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

