The Antiglycoxidative Ability of Selected Phenolic Compounds-An In Vitro Study
- PMID: 31344905
- PMCID: PMC6696369
- DOI: 10.3390/molecules24152689
The Antiglycoxidative Ability of Selected Phenolic Compounds-An In Vitro Study
Abstract
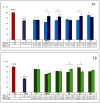
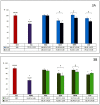
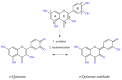
Hyperglycemia and oxidative stress may be observed in different diseases as important factors connected with their development. They often occur simultaneously and are considered together as one process: Glycoxidation. This can influence the function or structure of many macromolecules, for example albumin, by changing their physiological properties. This disturbs the homeostasis of the organism, so the search for natural compounds able to inhibit the glycoxidation process is a current and important issue. The aim of this study was the examination of the antiglycoxidative capacity of 16 selected phenolic compounds, belonging to three phenolic groups, as potential therapeutic agents. Their antiglycoxidative ability, in two concentrations (2 and 20 µM), were examined by in vitro study. The inhibition of the formation of both glycoxidative products (advanced glycation end products (AGEs) and advanced oxidation protein products (AOPPs)) were assayed. Stronger antiglycoxidative action toward the formation of both AOPPs and AGEs was observed for homoprotocatechuic and ferulic acids in lower concentrations, as well as catechin, quercetin, and 8-O-methylurolithin A in higher concentrations. Homoprotocatechuic acid demonstrated the highest antiglycoxidative capacity in both examined concentrations and amongst all of them. A strong, significant correlation between the percentage of AOPPs and AGEs inhibition by compounds from all phenolic groups, in both examined concentrations, was observed. The obtained results give an insight into the antiglycoxidative potential of phenolic compounds and indicate homoprotocatechuic acid to be the most promising antiglycoxidative agent, but further biological and pharmacological studies are needed.
Keywords: AGEs; AOPPs; antiglycoxidative potential; glycoxidation; phenolic compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

