Anti-Biofilm Property of Bioactive Upconversion Nanocomposites Containing Chlorin e6 against Periodontal Pathogens
- PMID: 31344909
- PMCID: PMC6695946
- DOI: 10.3390/molecules24152692
Anti-Biofilm Property of Bioactive Upconversion Nanocomposites Containing Chlorin e6 against Periodontal Pathogens
Abstract
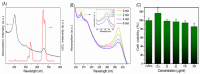
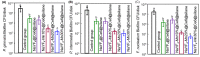
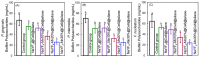
Photodynamic therapy (PDT) based periodontal disease treatment has received extensive attention. However, the deep tissue location of periodontal plaque makes the conventional PDT encounter a bottleneck. Herein, upconversion fluorescent nanomaterial with near-infrared light excitation was introduced into the treatment of periodontal disease, overcoming the limited tissue penetration depth of visible light in PDT. Photosensitizer Ce6 molecules were combined with upconversion nanoparticles (UCNPs) NaYF4:Yb,Er with a novel strategy. The hydrophobic UCNPs were modified with amphiphilic silane, utilizing the hydrophobic chain of the silane to bind to the hydrophobic groups of the UCNPs through a hydrophobic-hydrophobic interaction, and the Ce6 molecules were loaded in this hydrophobic layer. This achieves both the conversion of the hydrophobic to the hydrophilic surface and the loading of the oily photosensitizer molecules. Because the excitation position of the Ce6 molecule is in the red region, Mn ions were doped to enhance red light, and thus the improved PDT function. This Ce6 loaded UCNPs composites with efficient red upconversion luminescence show remarkable bacteriological therapeutic effect on Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum and the corresponding biofilms under 980 nm irradiation, indicating a high application prospect in the treatment of periodontal diseases.
Keywords: antibacterial photodynamic therapy; manganese ion; periodontitis; photosensitizer; upconversion nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

