Ocular-Component-Specific miRNA Expression in a Murine Model of Lens-Induced Myopia
- PMID: 31344984
- PMCID: PMC6695704
- DOI: 10.3390/ijms20153629
Ocular-Component-Specific miRNA Expression in a Murine Model of Lens-Induced Myopia
Abstract
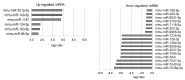
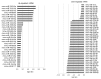
To identify tissues and molecules involved in refractive myopic shift and axial length elongation in a murine lens-induced myopia model, we performed a comprehensive analysis of microRNA (miRNA) expression. Three weeks after negative 30 diopter lens fixation on three-week-old C57BL/6J mice, total RNA was extracted from individual ocular components including cornea, iris, lens, retina, retinal pigment epithelium (RPE)/choroid, and sclera tissue. The miRNA expression analysis was pooled from three samples and carried out using Agilent Mouse miRNA Microarray (8 × 60 K) miRBase21.0. The expression ratio was calculated, and differentially expressed miRNAs were extracted, using GeneSpring GX 14.5. Myopic induction showed a significant myopic refractive change, axial elongation, and choroidal thinning. Through the comprehensive miRNA analysis, several upregulated miRNAs (56 in cornea tissue, 13 in iris tissue, 6 in lens tissue, 0 in retina tissue, 29 in RPE/choroid tissue, and 30 in sclera tissue) and downregulated miRNAs (7 in cornea tissue, 28 in iris tissue, 17 in lens tissue, 9 in retina tissue, 7 in RPE/choroid tissue, and 40 in sclera tissue) were observed. Overlapping expression changes in miRNAs were also found in different ocular components. Some of this miRNA dysregulation may be functionally involved in refractive myopia shift and axial length elongation.
Keywords: lens-induced myopia; miRNA; ocular components.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Yoshikawa M., Yamashiro K., Miyake M., Oishi M., Akagi-Kurashige Y., Kumagai K., Nakata I., Nakanishi H., Oishi A., Gotoh N., et al. Comprehensive replication of the relationship between myopia-related genes and refractive errors in a large Japanese cohort. Investig. Ophthalmol. Vis. Sci. 2014;55:7343–7354. doi: 10.1167/iovs.14-15105. - DOI - PubMed
-
- Verhoeven V.J., Hysi P.G., Wojciechowski R., Fan Q., Guggenheim J.A., Höhn R., Mac Gregor S., Hewitt A.W., Nag A., Cheng C.Y., et al. Genome-wide meta-analyses of multi-ethnic cohorts identify multiple new susceptibility loci for refractive error and myopia jointly conceived the project and supervised the work NIH public access author manuscript. Veluchamy A. Barathi. 2013;29:314–318.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

