Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial
- PMID: 31345462
- PMCID: PMC7041360
- DOI: 10.1016/S1473-3099(19)30127-6
Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial
Abstract
Background: Identifying new antifungals for cryptococcal meningitis is a priority given the inadequacy of current therapy. Sertraline has previously shown in vitro and in vivo activity against cryptococcus. We aimed to assess the efficacy and cost-effectiveness of adjunctive sertraline in adults with HIV-associated cryptococcal meningitis compared with placebo.
Methods: In this double-blind, randomised, placebo-controlled trial, we recruited HIV-positive adults with cryptococcal meningitis from two hospitals in Uganda. Participants were randomly assigned (1:1) to receive standard therapy with 7-14 days of intravenous amphotericin B (0·7-1·0 mg/kg per day) and oral fluconazole (starting at 800 mg/day) with either adjunctive sertraline or placebo. Sertraline was administered orally or via nasogastric tube at a dose of 400 mg/day for 2 weeks, followed by 200 mg/day for 12 weeks, then tapered off over 3 weeks. The primary endpoint was 18-week survival, analysed by intention-to-treat. This study is registered with ClinicalTrials.gov, number NCT01802385.
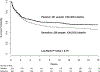
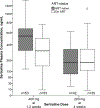
Findings: Between March 9, 2015, and May 29, 2017, we screened 842 patients with suspected meningitis and enrolled 460 of a planned 550 participants, at which point the trial was stopped for futility. Three patients in the sertraline group and three patients in the placebo group were lost to follow-up and therefore discontinued before study end. At 18 weeks, 120 (52%) of 229 patients in the sertraline group and 106 (46%) of 231 patients in the placebo group had died (hazard ratio 1·21, 95% CI 0·93-1·57; p=0·15). The fungal clearance rate from cerebrospinal fluid was similar between groups (0·43 -log10 CFU/mL per day [95% CI 0·37-0·50] in the sertraline group vs 0·47 -log10 CFU/mL per day [0·40-0·54] in the placebo group; p=0·59), as was occurrence of grade 4 or 5 adverse events (72 [31%] of 229 vs 75 [32%] of 231; p=0·98), most of which were associated with amphotericin B toxicity.
Interpretation: Sertraline did not reduce mortality and should not be used to treat patients with HIV-associated cryptococcal meningitis. The reasons for sertraline inactivity appear to be multifactorial and might be associated with insufficient duration of therapeutic sertraline concentrations.
Funding: National Institutes of Health and Medical Research Council, Wellcome Trust.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interests
All authors report no conflicts of interest to declare.
Figures
Comment in
-
HIV-associated cryptococcal meningitis: ongoing challenges and new opportunities.Lancet Infect Dis. 2019 Aug;19(8):793-794. doi: 10.1016/S1473-3099(19)30295-6. Lancet Infect Dis. 2019. PMID: 31345446 No abstract available.
References
-
- Nayak R, Xu J. Effects of sertraline hydrochloride and fluconazole combinations on Cryptococcus neoformans and Cryptococcus gattii. Mycology 2010; 1(2): 99–105.
-
- Trevino-Rangel Rde J, Villanueva-Lozano H, Hernandez-Rodriguez P, et al. Activity of sertraline against Cryptococcus neoformans: in vitro and in vivo assays. Med Mycol 2016; 54(3): 280–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

