Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial
- PMID: 31345626
- PMCID: PMC6722042
- DOI: 10.1016/S1470-2045(19)30395-X
Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial
Erratum in
-
Correction to Lancet Oncol 2019; 20: 1273-85.Lancet Oncol. 2019 Sep;20(9):e468. doi: 10.1016/S1470-2045(19)30526-1. Lancet Oncol. 2019. PMID: 31486369 Free PMC article. No abstract available.
Abstract
Background: The PORTEC-3 trial investigated the benefit of combined adjuvant chemotherapy and radiotherapy versus pelvic radiotherapy alone for women with high-risk endometrial cancer. We updated the analysis to investigate patterns of recurrence and did a post-hoc survival analysis.
Methods: In the multicentre randomised phase 3 PORTEC-3 trial, women with high-risk endometrial cancer were eligible if they had International Federation of Gynaecology and Obstetrics (FIGO) 2009 stage I, endometrioid grade 3 cancer with deep myometrial invasion or lymphovascular space invasion, or both; stage II or III disease; or stage I-III disease with serous or clear cell histology; were aged 18 years and older; and had a WHO performance status of 0-2. Participants were randomly assigned (1:1) to receive radiotherapy alone (48·6 Gy in 1·8 Gy fractions given on 5 days per week) or chemoradiotherapy (two cycles of cisplatin 50 mg/m2 given intravenously during radiotherapy, followed by four cycles of carboplatin AUC5 and paclitaxel 175 mg/m2 given intravenously), by use of a biased coin minimisation procedure with stratification for participating centre, lymphadenectomy, stage, and histological type. The co-primary endpoints were overall survival and failure-free survival. Secondary endpoints of vaginal, pelvic, and distant recurrence were analysed according to the first site of recurrence. Survival endpoints were analysed by intention-to-treat, and adjusted for stratification factors. Competing risk methods were used for failure-free survival and recurrence. We did a post-hoc analysis to analyse patterns of recurrence with 1 additional year of follow-up. The study was closed on Dec 20, 2013; follow-up is ongoing. This study is registered with ISRCTN, number ISRCTN14387080, and ClinicalTrials.gov, number NCT00411138.
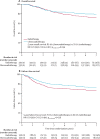
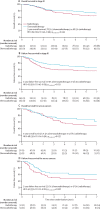
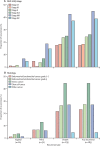
Findings: Between Nov 23, 2006, and Dec 20, 2013, 686 women were enrolled, of whom 660 were eligible and evaluable (330 in the chemoradiotherapy group, and 330 in the radiotherapy-alone group). At a median follow-up of 72·6 months (IQR 59·9-85·6), 5-year overall survival was 81·4% (95% CI 77·2-85·8) with chemoradiotherapy versus 76·1% (71·6-80·9) with radiotherapy alone (adjusted hazard ratio [HR] 0·70 [95% CI 0·51-0·97], p=0·034), and 5-year failure-free survival was 76·5% (95% CI 71·5-80·7) versus 69·1% (63·8-73·8; HR 0·70 [0·52-0·94], p=0·016). Distant metastases were the first site of recurrence in most patients with a relapse, occurring in 78 of 330 women (5-year probability 21·4%; 95% CI 17·3-26·3) in the chemoradiotherapy group versus 98 of 330 (5-year probability 29·1%; 24·4-34·3) in the radiotherapy-alone group (HR 0·74 [95% CI 0·55-0·99]; p=0·047). Isolated vaginal recurrence was the first site of recurrence in one patient (0·3%; 95% CI 0·0-2·1) in both groups (HR 0·99 [95% CI 0·06-15·90]; p=0·99), and isolated pelvic recurrence was the first site of recurrence in three women (0·9% [95% CI 0·3-2·8]) in the chemoradiotherapy group versus four (0·9% [95% CI 0·3-2·8]) in the radiotherapy-alone group (HR 0·75 [95% CI 0·17-3·33]; p=0·71). At 5 years, only one grade 4 adverse event (ileus or obstruction) was reported (in the chemoradiotherapy group). At 5 years, reported grade 3 adverse events did not differ significantly between the two groups, occurring in 16 (8%) of 201 women in the chemoradiotherapy group versus ten (5%) of 187 in the radiotherapy-alone group (p=0·24). The most common grade 3 adverse event was hypertension (in four [2%] women in both groups). At 5 years, grade 2 or worse adverse events were reported in 76 (38%) of 201 women in the chemoradiotherapy group versus 43 (23%) of 187 in the radiotherapy-alone group (p=0·002). Sensory neuropathy persisted more often after chemoradiotherapy than after radiotherapy alone, with 5-year rates of grade 2 or worse neuropathy of 6% (13 of 201 women) versus 0% (0 of 187). No treatment-related deaths were reported.
Interpretation: This updated analysis shows significantly improved overall survival and failure-free survival with chemoradiotherapy versus radiotherapy alone. This treatment schedule should be discussed and recommended, especially for women with stage III or serous cancers, or both, as part of shared decision making between doctors and patients. Follow-up is ongoing to evaluate long-term survival.
Funding: Dutch Cancer Society, Cancer Research UK, National Health and Medical Research Council, Project Grant, Cancer Australia Grant, Italian Medicines Agency, and the Canadian Cancer Society Research Institute.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Management of high-risk endometrial cancer: are we there yet?Lancet Oncol. 2019 Sep;20(9):1192-1193. doi: 10.1016/S1470-2045(19)30416-4. Epub 2019 Jul 22. Lancet Oncol. 2019. PMID: 31345628 No abstract available.
-
Clarifications sought for implications of PORTEC-3 in clinics.Lancet Oncol. 2019 Nov;20(11):e607. doi: 10.1016/S1470-2045(19)30534-0. Lancet Oncol. 2019. PMID: 31674312 No abstract available.
-
Radiation for Cancers of the Uterine Corpus and Cervix: Incremental Steps, and Glimmers of the Future.Int J Radiat Oncol Biol Phys. 2020 Nov 15;108(4):839-845. doi: 10.1016/j.ijrobp.2020.06.012. Int J Radiat Oncol Biol Phys. 2020. PMID: 33069345 No abstract available.
References
-
- Colombo N, Creutzberg C, Amant F. ESMO–ESGO–ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Radiother Oncol. 2015;117:559–581. - PubMed
-
- Straughn JM, Huh WK, Orr JW., Jr Stage IC adenocarcinoma of the endometrium: survival comparisons of surgically staged patients with and without adjuvant radiation therapy. Gynecol Oncol. 2003;89:295–300. - PubMed
-
- Creutzberg CL, van Putten WL, Warlam-Rodenhuis CC. Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: the Postoperative Radiation Therapy in Endometrial Carcinoma Trial. J Clin Oncol. 2004;22:1234–1241. - PubMed
-
- Bosse T, Peters EE, Creutzberg CL. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer—a pooled analysis of PORTEC 1 and 2 trials. Eur J Cancer. 2015;51:1742–1750. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

