Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer
- PMID: 31345636
- PMCID: PMC6853166
- DOI: 10.1016/j.eururo.2019.06.030
Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer
Abstract
Background: Prostate-specific membrane antigen (PSMA; folate hydrolase) prostate cancer (PC) expression has theranostic utility.
Objective: To elucidate PC PSMA expression and associate this with defective DNA damage repair (DDR).
Design, setting, and participants: Membranous PSMA (mPSMA) expression was scored immunohistochemically from metastatic castration-resistant PC (mCRPC) and matching, same-patient, diagnostic biopsies, and correlated with next-generation sequencing (NGS) and clinical outcome data.
Outcome measurements and statistical analysis: Expression of mPSMA was quantitated by modified H-score. Patient DNA was tested by NGS. Gene expression and activity scores were determined from mCRPC transcriptomes. Statistical correlations utilised Wilcoxon signed rank tests, survival was estimated by Kaplan-Meier test, and sample heterogeneity was quantified by Shannon's diversity index.
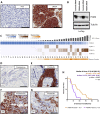
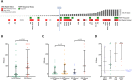
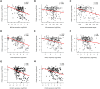
Results and limitations: Expression of mPSMA at diagnosis was associated with higher Gleason grade (p=0.04) and worse overall survival (p=0.006). Overall, mPSMA expression levels increased at mCRPC (median H-score [interquartile range]: castration-sensitive prostate cancer [CSPC] 17.5 [0.0-60.0] vs mCRPC 55.0 [2.8-117.5]). Surprisingly, 42% (n=16) of CSPC and 27% (n=16) of mCRPC tissues sampled had no detectable mPSMA (H-score <10). Marked intratumour heterogeneity of mPSMA expression, with foci containing no detectable PSMA, was observed in all mPSMA expressing CSPC (100%) and 37 (84%) mCRPC biopsies. Heterogeneous intrapatient mPSMA expression between metastases was also observed, with the lowest expression in liver metastases. Tumours with DDR had higher mPSMA expression (p=0.016; 87.5 [25.0-247.5] vs 20 [0.3-98.8]; difference in medians 60 [5.0-95.0]); validation cohort studies confirmed higher mPSMA expression in patients with deleterious aberrations in BRCA2 (p<0.001; median H-score: 300 [165-300]; difference in medians 195.0 [100.0-270.0]) and ATM (p=0.005; 212.5 [136.3-300]; difference in medians 140.0 [55.0-200]) than in molecularly unselected mCRPC biopsies (55.0 [2.75-117.5]). Validation studies using mCRPC transcriptomes corroborated these findings, also indicating that SOX2 high tumours have low PSMA expression.
Conclusions: Membranous PSMA expression is upregulated in some but not all PCs, with mPSMA expression demonstrating marked inter- and intrapatient heterogeneity. DDR aberrations are associated with higher mPSMA expression and merit further evaluation as predictive biomarkers of response for PSMA-targeted therapies in larger, prospective cohorts.
Patient summary: Through analysis of prostate cancer samples, we report that the presence of prostate-specific membrane antigen (PSMA) is extremely variable both within one patient and between different patients. This may limit the usefulness of PSMA scans and PSMA-targeted therapies. We show for the first time that prostate cancers with defective DNA repair produce more PSMA and so may respond better to PSMA-targeting treatments.
Keywords: BRCA2; Castration-resistant prostate cancer; Defective DNA repair; Prostate cancer; Prostate-specific membrane antigen; Theranostics; Treatment resistance; Tumour heterogeneity.
Copyright © 2019 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures

Comment in
-
Tumour Heterogeneity and Resistance to Therapy in Prostate Cancer: A Fundamental Limitation of Prostate-specific Membrane Antigen Theranostics or a Key Strength?Eur Urol. 2019 Oct;76(4):479-481. doi: 10.1016/j.eururo.2019.07.030. Epub 2019 Jul 25. Eur Urol. 2019. PMID: 31351666 No abstract available.
-
Re: Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer.Eur Urol. 2020 Apr;77(4):559-560. doi: 10.1016/j.eururo.2019.12.012. Epub 2019 Dec 23. Eur Urol. 2020. PMID: 31874727 No abstract available.
-
Editorial comment: the effect of neoadjuvant chemotherapy on tumour downstaging and outcomes in patients with muscle invasive bladder cancers.Transl Androl Urol. 2020 Jun;9(3):977-980. doi: 10.21037/tau-20-842. Transl Androl Urol. 2020. PMID: 32676377 Free PMC article. No abstract available.
References
-
- Yao V., Berkman C.E., Choi J.K., O'Keefe D.S., Bacich D.J. Expression of prostate-specific membrane antigen (PSMA), increases cell folate uptake and proliferation and suggests a novel role for PSMA in the uptake of the non-polyglutamated folate, folic acid. Prostate. 2010;70:305–316. - PubMed
-
- Tisman G. Modulation of one-carbon metabolism by B vitamins: implications for transformation and progression of prostate cancer. In: Spiess P.E., editor. Prostate cancer—from bench to bedside. IntechOpen; 2011.
-
- Silver D.A., Pellicer I., Fair W.R., Heston W.D., Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. - PubMed
-
- Bostwick D.G., Pacelli A., Blute M., Roche P., Murphy G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–2261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

