Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study
- PMID: 31345710
- PMCID: PMC6715822
- DOI: 10.1016/S1473-3099(19)30391-3
Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study
Abstract
Background: The emergence and spread of resistance in Plasmodium falciparum malaria to artemisinin combination therapies in the Greater Mekong subregion poses a major threat to malaria control and elimination. The current study is part of a multi-country, open-label, randomised clinical trial (TRACII, 2015-18) evaluating the efficacy, safety, and tolerability of triple artemisinin combination therapies. A very high rate of treatment failure after treatment with dihydroartemisinin-piperaquine was observed in Thailand, Cambodia, and Vietnam. The immediate public health importance of our findings prompted us to report the efficacy data on dihydroartemisinin-piperaquine and its determinants ahead of the results of the overall trial, which will be published later this year.
Methods: Patients aged between 2 and 65 years presenting with uncomplicated P falciparum or mixed species malaria at seven sites in Thailand, Cambodia, and Vietnam were randomly assigned to receive dihydroartemisinin-piperaquine with or without mefloquine, as part of the TRACII trial. The primary outcome was the PCR-corrected efficacy at day 42. Next-generation sequencing was used to assess the prevalence of molecular markers associated with artemisinin resistance (kelch13 mutations, in particular Cys580Tyr) and piperaquine resistance (plasmepsin-2 and plasmepsin-3 amplifications and crt mutations). This study is registered with ClinicalTrials.gov, number NCT02453308.
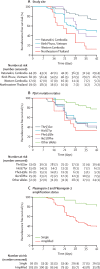
Findings: Between Sept 28, 2015, and Jan 18, 2018, 539 patients with acute P falciparum malaria were screened for eligibility, 292 were enrolled, and 140 received dihydroartemisinin-piperaquine. The overall Kaplan-Meier estimate of PCR-corrected efficacy of dihydroartemisinin-piperaquine at day 42 was 50·0% (95% CI 41·1-58·3). PCR-corrected efficacies for individual sites were 12·7% (2·2-33·0) in northeastern Thailand, 38·2% (15·9-60·5) in western Cambodia, 73·4% (57·0-84·3) in Ratanakiri (northeastern Cambodia), and 47·1% (33·5-59·6) in Binh Phuoc (southwestern Vietnam). Treatment failure was associated independently with plasmepsin2/3 amplification status and four mutations in the crt gene (Thr93Ser, His97Tyr, Phe145Ile, and Ile218Phe). Compared with the results of our previous TRACI trial in 2011-13, the prevalence of molecular markers of artemisinin resistance (kelch13 Cys580Tyr mutations) and piperaquine resistance (plasmepsin2/3 amplifications and crt mutations) has increased substantially in the Greater Mekong subregion in the past decade.
Interpretation: Dihydroartemisinin-piperaquine is not treating malaria effectively across the eastern Greater Mekong subregion. A highly drug-resistant P falciparum co-lineage is evolving, acquiring new resistance mechanisms, and spreading. Accelerated elimination of P falciparum malaria in this region is needed urgently, to prevent further spread and avoid a potential global health emergency.
Funding: UK Department for International Development, Wellcome Trust, Bill & Melinda Gates Foundation, Medical Research Council, and National Institutes of Health.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4·0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Accelerated evolution and spread of multidrug-resistant Plasmodium falciparum takes down the latest first-line antimalarial drug in southeast Asia.Lancet Infect Dis. 2019 Sep;19(9):916-917. doi: 10.1016/S1473-3099(19)30394-9. Epub 2019 Jul 22. Lancet Infect Dis. 2019. PMID: 31345711 Free PMC article. No abstract available.
-
Suboptimal dosing triggers artemisinin partner drug resistance.Lancet Infect Dis. 2019 Nov;19(11):1167-1168. doi: 10.1016/S1473-3099(19)30535-3. Lancet Infect Dis. 2019. PMID: 31657775 No abstract available.
-
Plasmodium falciparum resistance to piperaquine driven by PfCRT.Lancet Infect Dis. 2019 Nov;19(11):1168-1169. doi: 10.1016/S1473-3099(19)30543-2. Lancet Infect Dis. 2019. PMID: 31657776 Free PMC article. No abstract available.
-
Multigenic architecture of piperaquine resistance trait in Plasmodium falciparum.Lancet Infect Dis. 2020 Jan;20(1):26-27. doi: 10.1016/S1473-3099(19)30689-9. Lancet Infect Dis. 2020. PMID: 31876497 No abstract available.
References
-
- Nosten F, van Vugt M, Price R. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000;356:297–302. - PubMed
-
- WHO . World Health Organization; Geneva: 2018. World malaria report 2018.
-
- Dondorp AM, Smithuis FM, Woodrow C, Seidlein LV. How to contain artemisinin- and multidrug-resistant falciparum malaria. Trends Parasitol. 2017;33:353–363. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

