Anti-idiotypic antibodies elicit anti-HIV-1-specific B cell responses
- PMID: 31345931
- PMCID: PMC6780999
- DOI: 10.1084/jem.20190446
Anti-idiotypic antibodies elicit anti-HIV-1-specific B cell responses
Abstract
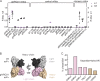
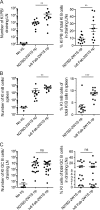
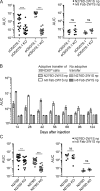
Human anti-HIV-1 broadly neutralizing antibodies (bNAbs) protect against infection in animal models. However, bNAbs have not been elicited by vaccination in diverse wild-type animals or humans, in part because B cells expressing the precursors of these antibodies do not recognize most HIV-1 envelopes (Envs). Immunogens have been designed that activate these B cell precursors in vivo, but they also activate competing off-target responses. Here we report on a complementary approach to expand specific B cells using an anti-idiotypic antibody, iv8, that selects for naive human B cells expressing immunoglobulin light chains with 5-amino acid complementarity determining region 3s, a key feature of anti-CD4 binding site (CD4bs)-specific VRC01-class antibodies. In mice, iv8 induced target cells to expand and mature in the context of a polyclonal immune system and produced serologic responses targeting the CD4bs on Env. In summary, the results demonstrate that an anti-idiotypic antibody can specifically recognize and expand rare B cells that express VRC01-class antibodies against HIV-1.
© 2019 Dosenovic et al.
Figures


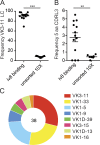

References
-
- Abbott R.K., Lee J.H., Menis S., Skog P., Rossi M., Ota T., Kulp D.W., Bhullar D., Kalyuzhniy O., Havenar-Daughton C., et al. 2018. Precursor Frequency and Affinity Determine B Cell Competitive Fitness in Germinal Centers, Tested with Germline-Targeting HIV Vaccine Immunogens. Immunity. 48:133–146.e6. 10.1016/j.immuni.2017.11.023 - DOI - PMC - PubMed
-
- Adams P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.W., Kapral G.J., Grosse-Kunstleve R.W., et al. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221. 10.1107/S0907444909052925 - DOI - PMC - PubMed
-
- Alam S.M., Aussedat B., Vohra Y., Meyerhoff R.R., Cale E.M., Walkowicz W.E., Radakovich N.A., Anasti K., Armand L., Parks R., et al. 2017. Mimicry of an HIV broadly neutralizing antibody epitope with a synthetic glycopeptide. Sci. Transl. Med. 9:eaai7521 10.1126/scitranslmed.aai7521 - DOI - PMC - PubMed
-
- Andrabi R., Voss J.E., Liang C.H., Briney B., McCoy L.E., Wu C.Y., Wong C.H., Poignard P., and Burton D.R.. 2015. Identification of Common Features in Prototype Broadly Neutralizing Antibodies to HIV Envelope V2 Apex to Facilitate Vaccine Design. Immunity. 43:959–973. 10.1016/j.immuni.2015.10.014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

