C-terminal proteolysis of the collagen VI α3 chain by BMP-1 and proprotein convertase(s) releases endotrophin in fragments of different sizes
- PMID: 31346034
- PMCID: PMC6746452
- DOI: 10.1074/jbc.RA119.008641
C-terminal proteolysis of the collagen VI α3 chain by BMP-1 and proprotein convertase(s) releases endotrophin in fragments of different sizes
Abstract
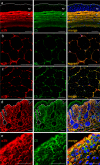
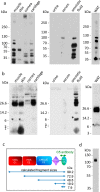
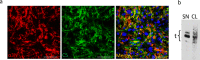
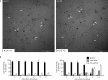
The assembly of collagen VI microfibrils is a multistep process in which proteolytic processing within the C-terminal globular region of the collagen VI α3 chain plays a major role. However, the mechanisms involved remain elusive. Moreover, C5, the short and most C-terminal domain of the α3 chain, recently has been proposed to be released as an adipokine that enhances tumor progression, fibrosis, inflammation, and insulin resistance and has been named "endotrophin." Serum endotrophin could be a useful biomarker to monitor the progression of such disorders as chronic obstructive pulmonary disease, systemic sclerosis, and kidney diseases. Here, using biochemical and isotopic MS-based analyses, we found that the extracellular metalloproteinase bone morphogenetic protein 1 (BMP-1) is involved in endotrophin release and determined the exact BMP-1 cleavage site. Moreover, we provide evidence that several endotrophin-containing fragments are present in various tissues and body fluids. Among these, a large C2-C5 fragment, which contained endotrophin, was released by furin-like proprotein convertase cleavage. By using immunofluorescence microscopy and EM, we also demonstrate that these proteolytic maturations occur after secretion of collagen VI tetramers and during microfibril assembly. Differential localization of N- and C-terminal regions of the collagen VI α3 chain revealed that cleavage products are deposited in tissue and cell cultures. The detailed information on the processing of the collagen VI α3 chain reported here provides a basis for unraveling the function of endotrophin (C5) and larger endotrophin-containing fragments and for refining their use as biomarkers of disease progression.
Keywords: BMP-1; Kunitz domain; Pro-C6; collagen; collagen VI; electron microscopy (EM); endotrophin; extracellular matrix protein; microfibrils; muscular dystrophy; proprotein convertase; protein processing.
© 2019 Heumüller et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

