NADPH-dependent extracellular superoxide production is vital to photophysiology in the marine diatom Thalassiosira oceanica
- PMID: 31346083
- PMCID: PMC6697786
- DOI: 10.1073/pnas.1821233116
NADPH-dependent extracellular superoxide production is vital to photophysiology in the marine diatom Thalassiosira oceanica
Abstract
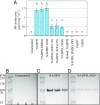
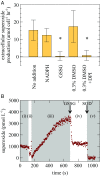
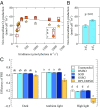
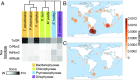
Reactive oxygen species (ROS) like superoxide drive rapid transformations of carbon and metals in aquatic systems and play dynamic roles in biological health, signaling, and defense across a diversity of cell types. In phytoplankton, however, the ecophysiological role(s) of extracellular superoxide production has remained elusive. Here, the mechanism and function of extracellular superoxide production by the marine diatom Thalassiosira oceanica are described. Extracellular superoxide production in T. oceanica exudates was coupled to the oxidation of NADPH. A putative NADPH-oxidizing flavoenzyme with predicted transmembrane domains and high sequence similarity to glutathione reductase (GR) was implicated in this process. GR was also linked to extracellular superoxide production by whole cells via quenching by the flavoenzyme inhibitor diphenylene iodonium (DPI) and oxidized glutathione, the preferred electron acceptor of GR. Extracellular superoxide production followed a typical photosynthesis-irradiance curve and increased by 30% above the saturation irradiance of photosynthesis, while DPI significantly impaired the efficiency of photosystem II under a wide range of light levels. Together, these results suggest that extracellular superoxide production is a byproduct of a transplasma membrane electron transport system that serves to balance the cellular redox state through the recycling of photosynthetic NADPH. This photoprotective function may be widespread, consistent with the presence of putative homologs to T. oceanica GR in other representative marine phytoplankton and ocean metagenomes. Given predicted climate-driven shifts in global surface ocean light regimes and phytoplankton community-level photoacclimation, these results provide implications for future ocean redox balance, ecological functioning, and coupled biogeochemical transformations of carbon and metals.
Keywords: biogeochemistry; oxidative stress; photosynthesis; reactive oxygen species.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lesser M. P., Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 68, 253–278 (2006). - PubMed
-
- Buetler T. M., Krauskopf A., Ruegg U. T., Role of superoxide as a signaling molecule. News Physiol. Sci. 19, 120–123 (2004). - PubMed
-
- Saran M., To what end does nature produce superoxide? NADPH oxidase as an autocrine modifier of membrane phospholipids generating paracrine lipid messengers. Free Radic. Res. 37, 1045–1059 (2003). - PubMed
-
- Hansel C. M., et al. , Dynamics of extracellular superoxide production by Trichodesmium colonies from the Sargasso Sea. Limnol. Oceanogr. 61, 1188–1200 (2016).

