FDA-ARGOS is a database with public quality-controlled reference genomes for diagnostic use and regulatory science
- PMID: 31346170
- PMCID: PMC6658474
- DOI: 10.1038/s41467-019-11306-6
FDA-ARGOS is a database with public quality-controlled reference genomes for diagnostic use and regulatory science
Abstract
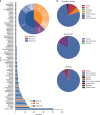
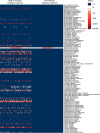
FDA proactively invests in tools to support innovation of emerging technologies, such as infectious disease next generation sequencing (ID-NGS). Here, we introduce FDA-ARGOS quality-controlled reference genomes as a public database for diagnostic purposes and demonstrate its utility on the example of two use cases. We provide quality control metrics for the FDA-ARGOS genomic database resource and outline the need for genome quality gap filling in the public domain. In the first use case, we show more accurate microbial identification of Enterococcus avium from metagenomic samples with FDA-ARGOS reference genomes compared to non-curated GenBank genomes. In the second use case, we demonstrate the utility of FDA-ARGOS reference genomes for Ebola virus target sequence comparison as part of a composite validation strategy for ID-NGS diagnostic tests. The use of FDA-ARGOS as an in silico target sequence comparator tool combined with representative clinical testing could reduce the burden for completing ID-NGS clinical trials.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Validation of Metagenomic Next-Generation Sequencing Tests for Universal Pathogen Detection.Arch Pathol Lab Med. 2017 Jun;141(6):776-786. doi: 10.5858/arpa.2016-0539-RA. Epub 2017 Feb 7. Arch Pathol Lab Med. 2017. PMID: 28169558
-
FDA's Activities Supporting Regulatory Application of "Next Gen" Sequencing Technologies.PDA J Pharm Sci Technol. 2014 Nov-Dec;68(6):626-30. doi: 10.5731/pdajpst.2014.01024. PDA J Pharm Sci Technol. 2014. PMID: 25475637
-
Comprehensive viral enrichment enables sensitive respiratory virus genomic identification and analysis by next generation sequencing.Genome Res. 2018 Jun;28(6):869-877. doi: 10.1101/gr.226316.117. Epub 2018 Apr 27. Genome Res. 2018. PMID: 29703817 Free PMC article.
-
Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection.Annu Rev Pathol. 2019 Jan 24;14:319-338. doi: 10.1146/annurev-pathmechdis-012418-012751. Epub 2018 Oct 24. Annu Rev Pathol. 2019. PMID: 30355154 Free PMC article. Review.
-
Next-Generation Sequencing for Infectious Disease Diagnosis and Management: A Report of the Association for Molecular Pathology.J Mol Diagn. 2015 Nov;17(6):623-34. doi: 10.1016/j.jmoldx.2015.07.004. Epub 2015 Sep 30. J Mol Diagn. 2015. PMID: 26433313 Review.
Cited by
-
Challenges for pathologists in implementing clinical microbiome diagnostic testing.J Pathol Clin Res. 2024 Sep;10(5):e70002. doi: 10.1002/2056-4538.70002. J Pathol Clin Res. 2024. PMID: 39289163 Free PMC article. Review.
-
Hospital sink traps as a potential source of the emerging multidrug-resistant pathogen Cupriavidus pauculus: characterization and draft genome sequence of strain MF1.J Med Microbiol. 2022 Feb;71(2):001501. doi: 10.1099/jmm.0.001501. J Med Microbiol. 2022. PMID: 35113779 Free PMC article.
-
SAUTE: sequence assembly using target enrichment.BMC Bioinformatics. 2021 Jul 21;22(1):375. doi: 10.1186/s12859-021-04174-9. BMC Bioinformatics. 2021. PMID: 34289805 Free PMC article.
-
FDA's proposed rule and its regulatory impact on emerging and reemerging neglected tropical diseases in the United States.PLoS Negl Trop Dis. 2024 May 9;18(5):e0012116. doi: 10.1371/journal.pntd.0012116. eCollection 2024 May. PLoS Negl Trop Dis. 2024. PMID: 38722919 Free PMC article. Review.
-
Laboratory validation of a clinical metagenomic next-generation sequencing assay for respiratory virus detection and discovery.Nat Commun. 2024 Nov 12;15(1):9016. doi: 10.1038/s41467-024-51470-y. Nat Commun. 2024. PMID: 39532844 Free PMC article.
References
-
- Snitkin Evan S., Won Sarah, Pirani Ali, Lapp Zena, Weinstein Robert A., Lolans Karen, Hayden Mary K. Integrated genomic and interfacility patient-transfer data reveal the transmission pathways of multidrug-resistant Klebsiella pneumoniae in a regional outbreak. Science Translational Medicine. 2017;9(417):eaan0093. doi: 10.1126/scitranslmed.aan0093. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

