Updating the evidence on drugs to treat overactive bladder: a systematic review
- PMID: 31346670
- PMCID: PMC6795617
- DOI: 10.1007/s00192-019-04022-8
Updating the evidence on drugs to treat overactive bladder: a systematic review
Abstract
Introduction: Overactive bladder (OAB) is a common condition, increasing with age and affecting quality of life. While numerous OAB drugs are available, persistence is low. We evaluated evidence published since 2012 to determine if newer drugs provided better efficacy and harm profiles.
Methods: We searched MEDLINE and the Cochrane Library from 2012 to September 2018 using terms for included drugs and requested information from manufacturers of included drugs. We performed dual review of all systematic review processes, evaluated study quality, and conducted meta-analyses using random effects models.
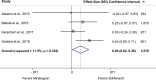
Results: In addition to 31 older studies, we included 20 trials published since 2012 (N = 16,478; 4 good, 11 fair, and 5 poor quality). Where statistical differences were found, they were clinically small (reductions of < 0.5 episodes/day). Solifenacin plus mirabegron improved efficacy outcomes over monotherapy with either drug, but significantly increased constipation compared with solifenacin and dry mouth compared with mirabegron. Solifenacin reduced incontinence over mirabegron and tolterodine and urgency episodes over tolterodine. Mirabegron did not differ from tolterodine in efficacy but had significantly lower incidence of dry mouth than solifenacin or tolterodine. Fesoterodine showed significant improvements but also anticholinergic effects vs. tolterodine. Oxybutynin, solifenacin, and tolterodine had similar efficacy, but dry mouth led to greater discontinuation with oxybutynin. Blurred vision, cardiac arrhythmia, and dizziness were uncommon.
Conclusion: New evidence confirms small, but clinically uncertain, differences among monotherapies and also between combination and monotherapy, regardless of statistical significance. While drugs mainly differed in incidence of dry mouth or constipation, none provided improved efficacy without increased harms.
Keywords: Antimuscarinics; Meta-analysis; Mirabegron; Overactive bladder; Systematic review; Urgency urinary incontinence.
Conflict of interest statement
Financial support statement: An earlier version of this systematic review was funded by the Drug Effectiveness Review Project. The funder participated in development of the scope and key questions, but had no role in selecting or analyzing studies. Updates to the earlier version were unfunded.
Figures


References
-
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. - DOI - PubMed
-
- Fantl J, Newman D, Colling J, DeLancey J, Keeys C, Loughery R, McDowell B, Norton P, Ouslander J, Schnelle J (1996) Urinary Incontinence in Adults: Acute and Chronic Management. Clinical Practice Guideline No. 2, 1996 Update (AHCPR Publication No. 96–0682). Rockville, MD: US Department of Health and Human Services. Public health service, Agency for health care policy and research.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

