Evaluating changes to Ralstonia pickettii in high-purity water to guide selection of potential calibration materials for online water bioburden analyzers
- PMID: 31346816
- PMCID: PMC6826051
- DOI: 10.1007/s10295-019-02192-4
Evaluating changes to Ralstonia pickettii in high-purity water to guide selection of potential calibration materials for online water bioburden analyzers
Abstract
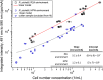
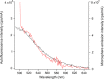
Online water bioburden analyzers (OWBAs) can provide real-time feedback on viable bacteria in high-purity water (HPW) systems for pharmaceutical manufacturers. To calibrate and validate OWBAs, which detect bacteria using scattered light and bacterial autofluorescence, standards are needed that mimic the characteristics of bacteria in HPW. To guide selection of potential standards, e.g., fluorescent microspheres, a relevant bacterial contaminant, Ralstonia pickettii, was characterized for size, count, viability, and autofluorescence after exposure for 24 h to HPW or a nutrient environment. The cells exposed to HPW showed smaller sizes, with lower counts and autofluorescence intensities, but similar spectral features. The cell characteristics are discussed in comparison with a set of fluorescent microspheres, considering factors relevant to OWBAs. These studies suggest that fluorescent microspheres should be relatively small (< 1 µm diameter) and dim, while covering a broad emission range from ≈ (420 to 600) nm to best mimic the representative R. pickettii.
Keywords: Autofluorescence; High-purity water; Online water bioburden analyzers; Ralstonia pickettii; Viability.
Figures




References
-
- Adley C, Ryan M, Pembroke J, Saieb FM. Biofilms: persistence and ubiquity. Powys: Biofilm Club; 2005. Ralstoniapickettii: biofilm formation in high-purity water; pp. 261–271.
-
- Alimova A, Katz A, Savage HE, Shah M, Minko G, Will DV, Rosen RB, McCormick SA, Alfano RR. Native fluorescence and excitation spectroscopic changes in Bacillus subtilis and Staphylococcus aureus bacteria subjected to conditions of starvation. Appl Opt. 2003;42:4080–4087. doi: 10.1364/AO.42.004080. - DOI - PubMed
-
- Commercial equipment and materials are identified in order to adequately specify certain procedures. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

