The Evolving Biomarker Landscape for Treatment Selection in Metastatic Colorectal Cancer
- PMID: 31347092
- PMCID: PMC6728290
- DOI: 10.1007/s40265-019-01165-2
The Evolving Biomarker Landscape for Treatment Selection in Metastatic Colorectal Cancer
Abstract
The approval of targeted therapies for metastatic colorectal cancer (mCRC) has led to important improvements in patient outcomes. However, it is still necessary to increase individualisation of treatments based on tumour genetic profiles to optimise efficacy, while minimising toxicity. As such, there is currently great focus on the discovery and validation of further biomarkers in mCRC, with many new potential prognostic and predictive markers being identified alongside developments in patient molecular profiling technologies. Here, we review data for validated and emerging biomarkers impacting treatment strategies in mCRC. We completed a structured literature search of the PubMed database to identify relevant publications, limiting for English-language publications published between 1 January 2014 and 11 July 2018. In addition, we performed a manual search of the key general oncology and CRC-focused congresses to identify abstracts reporting emerging mCRC biomarker data, and of ClinicalTrials.gov to identify ongoing clinical trials investigating emerging biomarkers in mCRC and/or molecular-guided clinical trials. There is solid evidence supporting the use of BRAF status as a prognostic biomarker and DYPD, UGT1A1, RAS, and microsatellite instability as predictive biomarkers in mCRC. There are a number of emerging biomarkers that may prove to be clinically relevant in the future to have prognostic (HPP1 methylation), predictive (HER3, microRNAs, anti-angiogenic markers, and CRC intrinsic subtypes), or both prognostic and predictive values (HER2, CpG island methylator phenotype, tumour mutational load, gene fusions, and consensus molecular subtypes). As such, new biomarker-led treatment strategies in addition to anti-epidermal growth factor receptor and anti-angiogenetic treatments are being explored. Biomarkers that are not recommended to be tested in clinical practice or are unlikely to be imminently clinically relevant for mCRC include thymidylate transferase, ERCC1, PIK3CA, and PTEN. We highlight the clinical utility of existing and emerging biomarkers in mCRC and provide recommended treatment strategies according to the biomarker status. An update on ongoing molecular-guided clinical trials is also provided.
Conflict of interest statement
Julien Taieb has acted in consultancy and/or advisory roles for, and received honoraria from, Amgen, Celgene, Lilly, Merck, MSD, Roche, Sanofi, Servier and Sirtex. Andreas Jung has acted in advisory roles for, and received honoraria and travel support from, Amgen, AstraZeneca, Biocartis, Boehringer Ingelheim, Bristol-Myers Squibb, Merck, Novartis and Roche. Andrea Sartore-Bianchi has acted in consultancy and/or advisory roles for, and received honoraria from, Amgen, Bayer, Lilly and Sanofi. Marc Peeters has received research funding from Amgen and Roche, and honoraria from Amgen, Lilly, Merck Serono, Remedus, Roche, Sanofi-Aventis, Servier, Sirtex and Terumo. Jenny Seligmann has acted in an advisory role for Roche and received honoraria from Merck Serono. Aziz Zaanan has acted in consultancy and/or advisory roles for Amgen, Baxter, Lilly, Merck Serono, MSD, Roche, Sanofi and Servier. Peter Burdon is an employee of Amgen (Europe) GmbH and owns shares in Amgen. Clara Montagut has acted in consultancy and/or advisory roles for Amgen, Merck Serono, Roche, Sanofi-Aventis, Servier and Symphogen, and received research funding from Amgen, Merck Serono and Symphogen. Pierre Laurent-Puig has acted in consultancy and/or advisory roles for Amgen, Biocartis, Boehringer Ingelheim, Bristol-Myers Squibb, Lilly, MDS, Merck Serono, Roche, Sanofi, and holds a patent for miR-31-3p.
Figures
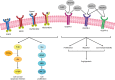
References
-
- Patil H, Saxena SG, Barrow CJ, Kanwar JR, Kapat A, Kanwar RK. Chasing the personalized medicine dream through biomarker validation in colorectal cancer. Drug Discov Today. 2017;22:111–119. - PubMed
-
- Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. - PubMed
-
- Boeckx N, Janssens K, Van Camp G, Rasschaert M, Papadimitriou K, Peeters M, et al. The predictive value of primary tumor location in patients with metastatic colorectal cancer: a systematic review. Crit Rev Oncol Hematol. 2018;121:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

