Negishi's Reagent Versus Rosenthal's Reagent in the Formation of Zirconacyclopentadienes
- PMID: 31347203
- PMCID: PMC6851999
- DOI: 10.1002/chem.201902255
Negishi's Reagent Versus Rosenthal's Reagent in the Formation of Zirconacyclopentadienes
Abstract
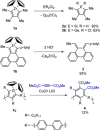
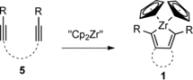
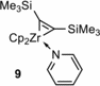
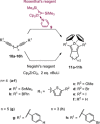
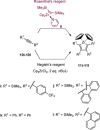
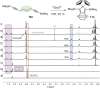
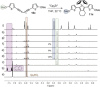
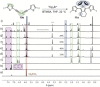
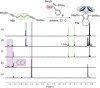
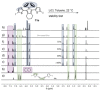
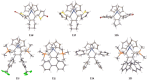
Zirconacyclopentadienes are versatile precursors for a large number of heteroles, which are accessible by Zr-element exchange reactions. The vast majority of reports describe their preparation by the use of Negishi's reagent, which is a species that is formed in situ. The zirconacyclopentadiene is then formed by the addition of one equivalent of a diyne or two equivalents of a monoyne moiety to this Negishi species. Another route involves Rosenthal's reagent (Cp2 Zr(py)Me3 SiC≡CSiMe3 ), which then reacts with a diyne or monoyne moiety. In this work, the efficiency of both routes was compared in terms of reaction time, stability of the product in the reaction mixture, and yield. The synthetic implications of using both routes are evaluated. Novel zirconacyclopentadienes were synthesized, characterized directly from the reaction mixture, and crystal structures could be obtained in most cases.
Keywords: Negishi′s reagent; Rosenthal′s reagent; metallacycles; substituent effects; zirconium.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Trautwein A. W., Süßmuth R. D., Jung G., Bioorg. Med. Chem. Lett. 1998, 8, 2381–2384. - PubMed
-
- None
-
- Wang C., Dong H., Hu W., Liu Y., Zhu D., Chem. Rev. 2012, 112, 2208–2267; - PubMed
-
- Facchetti A., Chem. Mater. 2011, 23, 733–758;
-
- Matano Y., Ohkubo H., Miyata T., Watanabe Y., Hayashi Y., Umeyama T., Imahori H., Eur. J. Inorg. Chem. 2014, 1620–1624.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

