A Sensitive and Selective Immunoassay for the Quantitation of Serum Latent Myostatin after In Vivo Administration of SRK-015, a Selective Inhibitor of Myostatin Activation
- PMID: 31347449
- PMCID: PMC6927069
- DOI: 10.1177/2472555219860779
A Sensitive and Selective Immunoassay for the Quantitation of Serum Latent Myostatin after In Vivo Administration of SRK-015, a Selective Inhibitor of Myostatin Activation
Abstract
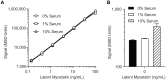
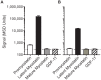
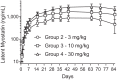
Myostatin, a member of the transforming growth factor β (TGFβ) superfamily, is a key regulator of skeletal muscle mass and a therapeutic target for muscle wasting diseases. We developed a human monoclonal antibody, SRK-015, that selectively binds to and inhibits proteolytic processing of myostatin precursors, thereby preventing growth factor release from the latent complex. As a consequence of antibody binding, latent myostatin accumulates in the circulation of animals treated with SRK-015 or closely related antibodies, suggesting that quantitation of latent myostatin in serum may serve as a biomarker for target engagement. To accurately measure SRK-015 target engagement, we developed a sensitive plate-based electrochemiluminescent immunoassay to quantitate latent myostatin in serum samples. The assay selectively recognizes latent myostatin without cross-reactivity to promyostatin, mature myostatin, or closely related members of the TGFβ superfamily. To enable use of the assay in samples from animals dosed with SRK-015, we incorporated a low-pH step that dissociates SRK-015 from latent myostatin, improving drug tolerance of the assay. The assay meets inter- and intra-assay accuracy and precision acceptance criteria, and it has a lower limit of quantitation (LLOQ) of 10 ng/mL. We then tested serum samples from a pharmacology study in cynomolgus monkeys treated with SRK-015. Serum latent myostatin increases after treatment with SRK-015, reaches a dose-dependent plateau approximately 20 days after dosing, and trends back toward baseline after cessation of antibody dosing. Taken together, these data suggest that this assay can be used to accurately measure levels of the primary circulating form of myostatin in population-based or pharmacodynamic studies.
Keywords: cynomolgus monkey; immunoassay; myostatin.
Conflict of interest statement
Figures


References
-
- Anderson S. B., Goldberg A. L., Whitman M. Identification of a Novel Pool of Extracellular Pro-Myostatin in Skeletal Muscle. J. Biol. Chem. 2008, 283, 7027–7035. - PubMed
-
- McPherron A. C., Lawler A. M., Lee S.-J. Regulation of Skeletal Muscle Mass in Mice by a New TGF-β Superfamily Member. Nature. 1997, 387, 83–90. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

