Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms
- PMID: 31347703
- PMCID: PMC6932943
- DOI: 10.1111/bph.14806
Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms
Abstract
Background and purpose: Acute pancreatitis (AP) is a common acute abdominal condition, frequently associated with intestinal barrier dysfunction, which aggravates AP retroactively. Butyrate exhibits anti-inflammatory effects in a variety of inflammatory diseases. However, its potential beneficial effect on AP and the underlying mechanisms have not been investigated.
Experimental approach: Experimental AP was induced by caerulein hyperstimulation in wild-type and GPR109A-/- mice. Sodium butyrate was administered intragastrically for 7 days prior to caerulein hyperstimulation. Anti-inflammatory mechanisms of butyrate were further investigated in peritoneal macrophages.
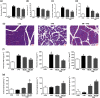
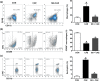
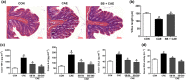
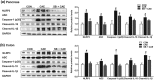
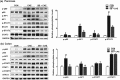
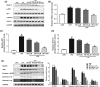
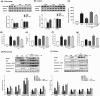
Key results: Butyrate prophylaxis attenuated AP as shown by reduced serum amylase and lipase levels, pancreatic oedema, myeloperoxidase activity, and improved pancreatic morphology. Amelioration of pancreatic damage by butyrate was associated with reduced levels of TNF-α, IL-6, and CCL2 and suppressed activation of the NLRP3 inflammasome in both pancreas and colon. Further, butyrate ameliorated pancreatic inflammation by suppressing interactions between histone deacetylase 1 (HDAC1) and AP1 and STAT1 with increased histone acetylation at H3K9, H3K14, H3K18, and H3K27 loci, resulting in suppression of NLRP3 inflammasome activation and modulation of immune cell infiltration in pancreas. Additionally, butyrate mediated STAT1/AP1-NLRP3 inflammasome suppression via HDAC1 inhibition was demonstrated in peritoneal macrophage. In colon, butyrate inhibited NLRP3 inflammasome activation via GPR109A. Accordingly, the modulatory effects of butyrate on AP, AP-associated gut dysfunction, and NLRP3 inflammasome activation were diminished in GPR109A-/- mice.
Conclusion and implications: Our study dissected tissue-specific anti-inflammatory mechanisms of butyrate during AP, suggesting that increased colonic levels of butyrate may be a strategy to protect against AP.
© 2019 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

