Myoarchitectural disarray of hypertrophic cardiomyopathy begins pre-birth
- PMID: 31347708
- PMCID: PMC6794206
- DOI: 10.1111/joa.13058
Myoarchitectural disarray of hypertrophic cardiomyopathy begins pre-birth
Abstract
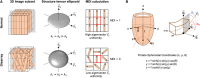
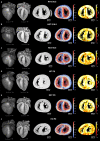
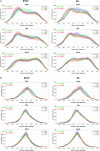
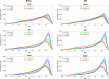
Myoarchitectural disarray - the multiscalar disorganisation of myocytes, is a recognised histopathological hallmark of adult human hypertrophic cardiomyopathy (HCM). It occurs before the establishment of left ventricular hypertrophy (LVH) but its early origins and evolution around the time of birth are unknown. Our aim is to investigate whether myoarchitectural abnormalities in HCM are present in the fetal heart. We used wild-type, heterozygous and homozygous hearts (n = 56) from a Mybpc3-targeted knock-out HCM mouse model and imaged the 3D micro-structure by high-resolution episcopic microscopy. We developed a novel structure tensor approach to extract, display and quantify myocyte orientation and its local angular uniformity by helical angle, angle of intrusion and myoarchitectural disarray index, respectively, immediately before and after birth. In wild-type, we demonstrate uniformity of orientation of cardiomyocytes with smooth transitions of helical angle transmurally both before and after birth but with traces of disarray at the septal insertion points of the right ventricle. In comparison, heterozygous mice free of LVH, and homozygous mice showed not only loss of the normal linear helical angulation transmural profiles observed in wild-type but also fewer circumferentially arranged myocytes at birth. Heterozygous and homozygous showed more disarray with a wider distribution than in wild-type before birth. In heterozygous mice, disarray was seen in the anterior, septal and inferior walls irrespective of stage, whereas in homozygous mice it extended to the whole LV circumference including the lateral wall. In conclusion, myoarchitectural disarray is detectable in the fetal heart of an HCM mouse model before the development of LVH.
Keywords: cardiac embryology; developmental biology; hypertrophic cardiomyopathy; myocardial disarray.
© 2019 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Adomian GE, Beazell J (1986) Myofibrillar disarray produced in normal hearts by chronic electrical pacing. Am Heart J 112, 79–83. - PubMed
-
- Ahrens J, Geveci B, Law C (2005) ParaView: an end‐user tool for large‐data visualization In: Visualization Handbook. (eds Hansen CD, Johnson CR), pp. 717–732, Elsevier.
-
- Ammirati E, Contri R, Coppini R, et al. (2016) Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Fail 18, 1106–1118. - PubMed
-
- Baličević V, Lončarić S, Cárdenes R, et al. (2015) Assessment of Myofiber Orientation in High Resolution Phase‐Contrast CT Images, pp. 111–119. Cham: Springer.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

