Caspase-3-mediated GSDME activation contributes to cisplatin- and doxorubicin-induced secondary necrosis in mouse macrophages
- PMID: 31347748
- PMCID: PMC6797504
- DOI: 10.1111/cpr.12663
Caspase-3-mediated GSDME activation contributes to cisplatin- and doxorubicin-induced secondary necrosis in mouse macrophages
Abstract
Objective: Induction of secondary necrosis/pyroptosis contributes to the toxicity of chemotherapeutic drugs, in which gasdermin E (GSDME) plays critical roles. This study aimed to explore whether GSDME is involved in mediating the cytotoxic effects of cisplatin and doxorubicin on mouse macrophages.
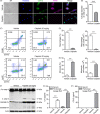
Methods: RAW 264.7 cells and bone marrow-derived macrophages (BMDMs) were treated with cisplatin or doxorubicin. Propidium iodide staining was used to assay necrosis, and immunoblotting was performed to detect protein expression. GSDME was knocked down by using small interfering RNA. Mice were injected intraperitoneally to evaluate toxicity to macrophages in vivo. Flow cytometry and immunofluorescence microscopy were adopted to analyse phenotypes of peritoneal cells. Cytokine levels were assayed by cytometric bead array.
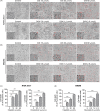
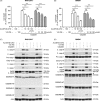
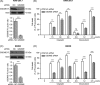
Results: Both cisplatin and doxorubicin dose-dependently induced necrosis in mouse RAW 264.7 macrophages and BMDMs. Accompanying this, multiple caspases were activated, concomitant with the cleavage of poly (ADP-ribose) polymerase. Consistent with caspase-3 activation, GSDME was cleaved to generate its N-terminal fragment (GSDME-NT), thus leading to secondary necrosis/pyroptosis. Inhibition of caspase-3 significantly attenuated the generation of GSDME-NT concurrently with decreased necrosis in macrophages. GSDME knockdown also evidently decreased the necrosis in RAW 264.7 and BMDMs. Besides, cisplatin administration depleted peritoneal macrophages in mice, which was associated with caspase-3 activation and GSDME-NT generation. Consistent with the macrophage depletion, cisplatin administration significantly decreased survival of mice with bacterial infection.
Conclusion: Chemotherapeutic cisplatin and doxorubicin exerted their cytotoxicity on macrophages partly by inducing caspase-3/GSDME-mediated secondary necrosis.
Keywords: caspase-3; chemotherapeutic drugs; gasdermin E; macrophages; secondary necrosis.
© 2019 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Cheung‐Ong K, Giaever G, Nislow C. DNA‐damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem Biol. 2013;20:648‐659. - PubMed
-
- Roos WP, Kaina B. DNA damage‐induced cell death by apoptosis. Trends Mol Med. 2006;12:440‐450. - PubMed
-
- Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase‐3 cleavage of a gasdermin. Nature. 2017;547:99‐103. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

