Efficacy and safety of nivolumab in Japanese patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma: Multicenter, open-label phase 2 trial
- PMID: 31348579
- PMCID: PMC6726684
- DOI: 10.1111/cas.14148
Efficacy and safety of nivolumab in Japanese patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma: Multicenter, open-label phase 2 trial
Abstract
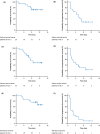
Nivolumab is a human monoclonal antibody against the immune checkpoint receptor programmed death-1, inhibiting binding to programmed death-ligand 1 or 2 (PD-L1 or PD-L2). This phase 2 study evaluated the efficacy and safety of nivolumab in patients with advanced/recurrent uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma (STS). Patients received nivolumab 240 mg at 2-week intervals. Primary endpoint was objective response rate; secondary endpoints included overall survival, progression-free survival, and safety. PD-L1 expression and microsatellite-instability (MSI) status were analyzed as potential efficacy biomarkers. Objective response rate was 25%, 23%, and 0% in patients with cervical cancer (n = 20), corpus cancer (n = 22), and STS (n = 21), respectively. The lower 80% confidence intervals of objective response rates in patients with cervical or corpus cancer exceeded the threshold rate (5%); the primary endpoint was met in cervical and corpus cancer, but not in STS. Median progression-free survival was 5.6, 3.4, and 1.4 months, and 6-month overall survival was 84%, 73%, and 86% in cervical cancer, corpus cancer, and STS, respectively. The objective response rate was higher in patients with cervical cancer with PD-L1-positive (n = 5/15; 33%) versus PD-L1-negative (n = 0/5; 0%) tumors. The two patients with corpus cancer classified as MSI-high responded; the six patients classified as microsatellite stable did not respond. Overall, nivolumab showed acceptable toxicity in all cohorts, with evidence of clinical activity in uterine cervical or corpus cancer, but not in STS. PD-L1 expression in cervical cancer and MSI-high in corpus cancer may predict clinical activity of nivolumab in these cancers.
Keywords: nivolumab; programmed death-1; soft tissue sarcoma; uterine cervical cancer; uterine corpus cancer.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
K. Tamura has received research funding from Ono Pharmaceutical, AstraZeneca, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly, Kyowa Pharmaceutical Inc., MSD, Novartis, and Pfizer. K. Hasegawa has received honoraria from Chugai Pharmaceutical. N. Katsumata has no relationships to disclose. K. Matsumoto has received research funding from Ono Pharmaceutical, MSD, and Novartis and honoraria from Chugai Pharmaceutical and Kyowa Hakko Kirin. H. Mukai has received honoraria from AstraZeneca, Pfizer, and Taiho Pharmaceutical. S. Takahashi has received research funding from Ono Pharmaceutical, AstraZeneca, Daiichi Sankyo, Eisai, IQVIA, MSD, Quintiles, and Taiho Pharmaceutical and honoraria from Bayer, Bristol‐Myers Squibb, Eisai, and Taiho Pharmaceutical. H. Nomura has no relationships to disclose. H. Minami has received institutional research funding from Ono Pharmaceutical, Astellas Pharma, Bristol‐Myers Squibb, Chugai Pharmaceutical, Dainippon Sumitomo Pharma, Eisai, Eli Lilly, Kyowa Hakko Kirin, Novartis, Sanofi, and Taiho Pharmaceutical and honoraria from Ono Pharmaceutical, Bayer, Bristol‐Myers Squibb, Celgene, Chugai Pharmaceutical, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eisai, Eli Lilly, Janssen, Kowa Pharmaceutical, Kyowa Hakko Kirin, Merck Serono, Novartis, Otsuka Pharmaceutical, Pfizer, Sanofi, Shire, Taiho Pharmaceutical, and Takeda Pharmaceutical, and participated in consultancies for Ono Pharmaceutical and Merck Serono. This work was supported by Ono Pharmaceutical and Bristol‐Myers Squibb. The study sponsor was involved in study design, writing of the report, and in the decision to submit the article for publication. Study drug was provided by Ono Pharmaceutical. AC Medical Inc. and A2 Healthcare Corporation collected and analyzed the data in conjunction with the Ono Pharmaceutical Data Management and Statistics groups. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Figures



References
-
- Frenel JS, Le Tourneau C, O'Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1‐positive cervical cancer: results from the phase Ib KEYNOTE‐028 trial. J Clin Oncol. 2017;35:4035‐4041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

