Assembling a Natural Small Molecule into a Supramolecular Network with High Structural Order and Dynamic Functions
- PMID: 31348651
- PMCID: PMC6696886
- DOI: 10.1021/jacs.9b05740
Assembling a Natural Small Molecule into a Supramolecular Network with High Structural Order and Dynamic Functions
Abstract
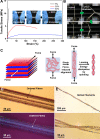
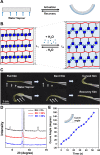
Programming the hierarchical self-assembly of small molecules has been a fundamental topic of great significance in biological systems and artificial supramolecular systems. Precise and highly programmed self-assembly can produce supramolecular architectures with distinct structural features. However, it still remains a challenge how to precisely control the self-assembly pathway in a desirable way by introducing abundant structural information into a limited molecular backbone. Here we disclose a strategy that directs the hierarchical self-assembly of sodium thioctate, a small molecule of biological origin, into a highly ordered supramolecular layered network. By combining the unique dynamic covalent ring-opening-polymerization of sodium thioctate and an evaporation-induced interfacial confinement effect, we precisely direct the dynamic supramolecular self-assembly of this simple small molecule in a scheduled hierarchical pathway, resulting in a layered structure with long-range order at both macroscopic and molecular scales, which is revealed by small-angle and wide-angle X-ray scattering technologies. The resulting supramolecular layers are found to be able to bind water molecules as structural water, which works as an interlayer lubricant to modulate the material properties, such as mechanical performance, self-healing capability, and actuating function. Analogous to many reversibly self-assembled biological systems, the highly dynamic polymeric network can be degraded into monomers and reformed by a water-mediated route, exhibiting full recyclability in a facile, mild, and environmentally friendly way. This approach for assembling commercial small molecules into structurally complex materials paves the way for low-cost functional supramolecular materials based on synthetically simple procedures.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Lutz J. F.; Lehn J. M.; Meijer E. W.; Matyjaszewski K. From precision polymers to complex materials and systems. Nat. Rev. Mater. 2016, 1, 16024.10.1038/natrevmats.2016.24. - DOI
-
- Van Hameren R.; Schön P.; Van Buul A. M.; Hoogboom J.; Lazarenko S. V.; Gerritsen J. W.; Engelkamp H.; Christianen P. C. M.; Heus H. A.; Maan J. C.; Rasing T.; Speller S.; Rowan A. E.; Elemans J. A. A. W.; Nolte R. J. M. Macroscopic hierarchical surface patterning of porphyrin trimers via self-assembly and dewetting. Science 2006, 314, 1433–1436. 10.1126/science.1133004. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

