Toxoplasma F-box protein 1 is required for daughter cell scaffold function during parasite replication
- PMID: 31348812
- PMCID: PMC6685633
- DOI: 10.1371/journal.ppat.1007946
Toxoplasma F-box protein 1 is required for daughter cell scaffold function during parasite replication
Abstract
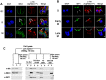
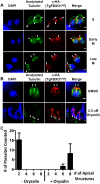
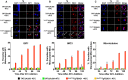
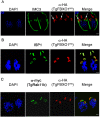
By binding to the adaptor protein SKP1 and serving as substrate receptors for the SKP1 Cullin, F-box E3 ubiquitin ligase complex, F-box proteins regulate critical cellular processes including cell cycle progression and membrane trafficking. While F-box proteins are conserved throughout eukaryotes and are well studied in yeast, plants, and animals, studies in parasitic protozoa are lagging. We have identified eighteen putative F-box proteins in the Toxoplasma genome of which four have predicted homologs in Plasmodium. Two of the conserved F-box proteins were demonstrated to be important for Toxoplasma fitness and here we focus on an F-box protein, named TgFBXO1, because it is the most highly expressed by replicative tachyzoites and was also identified in an interactome screen as a Toxoplasma SKP1 binding protein. TgFBXO1 interacts with Toxoplasma SKP1 confirming it as a bona fide F-box protein. In interphase parasites, TgFBXO1 is a component of the Inner Membrane Complex (IMC), which is an organelle that underlies the plasma membrane. Early during replication, TgFBXO1 localizes to the developing daughter cell scaffold, which is the site where the daughter cell IMC and microtubules form and extend from. TgFBXO1 localization to the daughter cell scaffold required centrosome duplication but before kinetochore separation was completed. Daughter cell scaffold localization required TgFBXO1 N-myristoylation and was dependent on the small molecular weight GTPase, TgRab11b. Finally, we demonstrate that TgFBXO1 is required for parasite growth due to its function as a daughter cell scaffold effector. TgFBXO1 is the first F-box protein to be studied in apicomplexan parasites and represents the first protein demonstrated to be important for daughter cell scaffold function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Dubey JP. History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol. 2009;39(8):877–82. Epub 2009/07/25. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

