Does phenotypic plasticity initiate developmental bias?
- PMID: 31348849
- PMCID: PMC7004013
- DOI: 10.1111/ede.12304
Does phenotypic plasticity initiate developmental bias?
Abstract
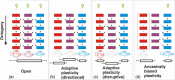
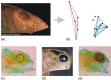
The generation of variation is paramount for the action of natural selection. Although biologists are now moving beyond the idea that random mutation provides the sole source of variation for adaptive evolution, we still assume that variation occurs randomly. In this review, we discuss an alternative view for how phenotypic plasticity, which has become well accepted as a source of phenotypic variation within evolutionary biology, can generate nonrandom variation. Although phenotypic plasticity is often defined as a property of a genotype, we argue that it needs to be considered more explicitly as a property of developmental systems involving more than the genotype. We provide examples of where plasticity could be initiating developmental bias, either through direct active responses to similar stimuli across populations or as the result of programmed variation within developmental systems. Such biased variation can echo past adaptations that reflect the evolutionary history of a lineage but can also serve to initiate evolution when environments change. Such adaptive programs can remain latent for millions of years and allow development to harbor an array of complex adaptations that can initiate new bouts of evolution. Specifically, we address how ideas such as the flexible stem hypothesis and cryptic genetic variation overlap, how modularity among traits can direct the outcomes of plasticity, and how the structure of developmental signaling pathways is limited to a few outcomes. We highlight key questions throughout and conclude by providing suggestions for future research that can address how plasticity initiates and harbors developmental bias.
Keywords: cryptic genetic variation; developmental signaling pathways; flexible stem; plasticity integration.
© 2019 The Authors. Evolution & Development Published by Wiley Periodicals, Inc.
Figures

References
-
- Andersson, J. (2003). Effects of diet‐induced resource polymorphism on performance in arctic charr (Salvelinus alpinus). Evolutionary Ecology Research, 5, 213–228.
-
- Bailey, S. F. , Rodrigue, N. , & Kassen, R. (2015). The effect of selection environment on the probability of parallel evolution. Molecular Biology and Evolution, 32, 1436–1448. - PubMed
-
- Bondurianskay, R. , & Day, T. (2018). Extended heredity: a new understanding of inheritance and evolution. Princeton, NJ: Princeton University Press.
-
- Callahan, H. S. , & Pigliucci, M. (2002). Shade‐induced plasticity and its ecological significance in wild populations of Arabidopsis thaliana . Ecology, 83, 1965–1980.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

